Characterising the asynchronous resurgence of common respiratory viruses following the COVID-19 pandemic
- PMID: 39948338
- PMCID: PMC11825952
- DOI: 10.1038/s41467-025-56776-z
Characterising the asynchronous resurgence of common respiratory viruses following the COVID-19 pandemic
Abstract
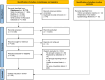
The COVID-19 pandemic and relevant non-pharmaceutical interventions (NPIs) interrupted the circulation of common respiratory viruses. These viruses demonstrated an unprecedented asynchronous resurgence as NPIs were relaxed. We compiled a global dataset from a systematic review, online surveillance reports and unpublished data from Respiratory Virus Global Epidemiology Network, encompassing 92 sites. We compared the resurgence timings of respiratory viruses within each site and synthesised differences in timings across sites, using a generalised linear mixed-effects model. We revealed a distinct sequential timing in the first post-pandemic resurgence: rhinovirus resurged the earliest, followed by seasonal coronavirus, parainfluenza virus, respiratory syncytial virus, adenovirus, metapneumovirus and influenza A virus, with influenza B virus exhibiting the latest resurgence. Similar sequential timing was observed in the second resurgence except influenza A virus caught up with metapneumovirus. The consistent asynchrony across geographical regions suggests that virus-specific characteristics, rather than location-specific factors, determining the relative timing of resurgence.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: T.Z. reports personal fees from Pfizer, outside the submitted work. D.-J.N. reports grants from Wellcome Trust related to the submitted manuscript. C.M.-A. reports personal fees from MSD, Pfizer and Sanofi, and travel grants from MSD and Pfizer, outside the submitted work. J.-W.K. reports grants from Centers for Disease Control and Prevention (CDC) related to the submitted work, grants from National Institutes of Health, Merck Sharp and Dohme Corporation, United States Department of Agriculture, National Science Foundation, CDC and Greenwall Foundation, and participation on data safety monitoring boards for Enhancing the Diabetes Prevention Program to promote weight loss among non-responders in a community based lifestyle intervention funded by National Institute of Diabetes and Digestive and Kidney Diseases, outside the submitted work. J.-M.L. reports that her employer received grants for the conduct of clinical research from Pfizer, GSK, Sanofi, Merck, and Moderna and personal fees paid to her employer from Enanta, GSK and Sanofi. T.H. reports personal fees from MSD, Pfizer and Sanofi, and participation on data safety monitoring boards for Sanofi, Enanta, MSD, Moderna, Shionogi and Pfizer, outside the submitted work. H.N. reports grants from the Innovative Medicines Initiative, WHO, the National Institute for Health Research, Pfizer, and Icosavax; and personal fees from the Gates Foundation, Pfizer, ReViral, GSK, Merck, Icosavax, Sanofi, Novavax, and AbbVie, outside the submitted work. X.W reports grants from GSK and personal fees from Pfizer, outside the submitted work. Y.L. reports grants from WHO, the Wellcome Trust, and GSK, and personal fees from Pfizer, outside the submitted work. All other authors declare no competing interests.
Figures





References
-
- GBD 2016 Lower Respiratory Infections Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis.18, 1191–1210 (2018). - PMC - PubMed
-
- Li, Y. et al. Global patterns in monthly activity of influenza virus, respiratory syncytial virus, parainfluenza virus, and metapneumovirus: a systematic analysis. Lancet Glob. Health7, e1031–e1045 (2019). - PubMed
-
- Li, Y., Campbell, H., Nair, H. & RESCEU Investigators. Unveiling the risk period for death after respiratory syncytial virus illness in young children using a self-controlled case series design. J. Infect. Dis.222, S634–S639 (2020). - PubMed
-
- Moriyama, M., Hugentobler, W. J. & Iwasaki, A. Seasonality of respiratory viral infections. Annu. Rev. Virol.7, 83–101 (2020). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

