Activin E is a new guardian protecting against hepatic steatosis via inhibiting lipolysis in white adipose tissue
- PMID: 39948368
- PMCID: PMC11873131
- DOI: 10.1038/s12276-025-01403-6
Activin E is a new guardian protecting against hepatic steatosis via inhibiting lipolysis in white adipose tissue
Abstract
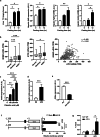
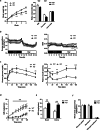
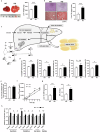
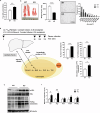
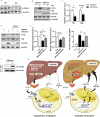
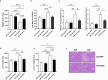
Hepatic endoplasmic reticulum (ER) stress is implicated in the development of steatosis and its progression to nonalcoholic steatohepatitis (NASH). The ER in the liver can sustain metabolic function by activating defense mechanisms that delay or prevent the progression of nonalcoholic fatty liver disease (NAFLD). However, the precise mechanisms by which the ER stress response protects against NAFLD remain largely unknown. Recently, activin E has been linked to metabolic diseases such as insulin resistance and NAFLD. However, the physiological conditions and regulatory mechanisms driving hepatic Inhbe expression (which encodes activin E) as well as the metabolic role of activin E in NAFLD require further investigation. Here we found that hepatic Inhbe expression increased under prolonged fasting and ER stress conditions, which was mediated by ATF4, as determined by promoter analysis in a mouse model. Consistently, a positive correlation between INHBE and ATF4 expression levels in relation to NAFLD status was confirmed using public human NAFLD datasets. To investigate the role of activin E in hepatic steatosis, we assessed the fluxes of the lipid metabolism in an Inhbe-knockout mouse model. These mice displayed a lean phenotype but developed severe hepatic steatosis under a high-fat diet. The deficiency of Inhbe resulted in increased lipolysis in adipose tissue, leading to increased fatty acid influx into the liver. Conversely, hepatic overexpression of Inhbe ameliorated hepatic steatosis by suppressing lipolysis in adipose tissue through ALK7-Smad signaling. In conclusion, activin E serves as a regulatory hepatokine that prevents fatty acid influx into the liver, thereby protecting against NAFLD.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: I.-Y.K. and S.P. are shareholders in Myocare, Inc. The other authors have no conflicts of interest to report.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

