Portimine A toxin causes skin inflammation through ZAKα-dependent NLRP1 inflammasome activation
- PMID: 39948420
- PMCID: PMC11903881
- DOI: 10.1038/s44321-025-00197-4
Portimine A toxin causes skin inflammation through ZAKα-dependent NLRP1 inflammasome activation
Abstract
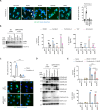
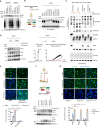
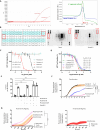
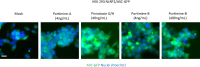
In 2020-2021, a "mysterious illness" struck Senegalese fishermen, causing severe acute dermatitis in over one thousand individuals following exposure through drift-net fishing activity. Here, by performing deep analysis of the environmental samples we reveal the presence of the marine dinoflagellate Vulcanodinium rugosum and its associated cyclic imine toxins. Specifically, we show that the toxin PortimineA, strongly enriched in environmental samples, impedes ribosome function in human keratinocytes, which subsequently activates the stress kinases ZAKα and P38 and promotes the nucleation of the human NLRP1 inflammasome, leading to the release of IL-1β/IL-18 pro-inflammatory cytokines and cell death. Furthermore, cell-based models highlight that naturally occurring mutations in the P38-targeted sites of human NLRP1 are unable to respond to PortimineA exposure. Finally, the development and use of human organotypic skins and zebrafish models of PortimineA exposure demonstrate that the ZAKα-NLRP1 axis drives skin necrosis and inflammation. Our results exemplify the threats to human health caused by emerging environmental toxins and identify ZAKα and NRLP1 as important pharmacological targets to mitigate PortimineA toxicity.
Keywords: Environmental Toxins; NLRP1 Inflammasome; Ribotoxic Stress Response; Skin Pathology.
© 2025. The Author(s).
Conflict of interest statement
Disclosure and competing interests statement. The authors declare no competing interests.
Figures



References
-
- Abadie E, Chiantella C, Crottier A, Rhodes L, Masseret E, Berteaux T, Laabir M (2018) What are the main environmental factors driving the development of the neurotoxic dinoflagellate Vulcanodinium rugosum in a Mediterranean ecosystem (Ingril lagoon, France)? Harmful Algae 75:75–86 - PubMed
-
- Bauernfried S, Scherr MJ, Pichlmair A, Duderstadt KE, Hornung V (2021) Human NLRP1 is a sensor for double-stranded RNA. Science 371:eabd0811 - PubMed
MeSH terms
Substances
Grants and funding
- INFLAME 804249/EC | ERC | HORIZON EUROPE European Research Council (ERC)
- PSICOPAK/Agence Nationale de la Recherche (ANR)
- INFLAMATOX/Agence Nationale de la Recherche (ANR)
- COMETH-/Agence Nationale de la Recherche (ANR)
- AJE20151034460/Fondation pour la Recherche Médicale (FRM)
- CIFRE/Association Nationale de la Recherche et de la Technologie (ANRT)
- LCF/PR/HR21/52410027/la Caixa' Foundation
- PID2022-139754OB-I00/'la Caixa' Foundation ('la Caixa')
- RYC2019-571 027799-I/'la Caixa' Foundation ('la Caixa')
- 01DG12073E/Institut de Recherche pour le Développement (IRD)
- H22J1a0040/Asian Skin Microbiome Programme 2.0 IAF-PP
- MOH-001499/MOH | National Medical Research Council (NMRC)
- RT23/23/Ministry of Education Tier 1 grant
- MOE-T2EP30222-0008/Ministry of Education Tier 2
- PHYTOX-R306-01/Institut Français de Recherche pour l'Exploitation de la Mer (IFREMER)
- Awatox/Art Sunu Gueej initiative/01DG12073E/Institut Français de Recherche pour l'Exploitation de la Mer (IFREMER)
LinkOut - more resources
Full Text Sources

