Electrolyte and metabolite composition of cystic fluid from a rat model of ARPKD
- PMID: 39948436
- PMCID: PMC11825955
- DOI: 10.1038/s42003-025-07631-w
Electrolyte and metabolite composition of cystic fluid from a rat model of ARPKD
Abstract
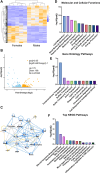
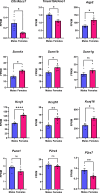
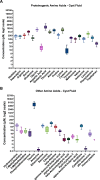
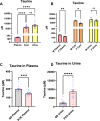
Fluid-filled cysts are the key feature of polycystic kidney disease, which eventually leads to renal failure. We analyzed the composition of cyst fluid from a rat model of autosomal recessive polycystic kidney disease, the PCK rat, and identified sexual differences. Our results demonstrate that the ion composition of cyst fluid differs from that of urine or plasma. Untargeted metabolomics combined with transcriptomic data identified tryptophan metabolism, enzyme metabolism, steroid hormone biosynthesis, and fatty acid metabolism as pathways differing between male and female PCK rats. We quantified 42 amino acids in the cyst fluid (PCK only), plasma, and urine of male and female PCK rats and Sprague Dawley rats. Taurine was the most concentrated amino acid present in the cyst fluid, and PCK rat urinary taurine excretion was over 3-fold greater than Sprague Dawley rats. Understanding the composition of cyst fluid provides valuable insights into disease pathophysiology and may help identify potential dietary or pharmacological interventions to mitigate disease progression and improve patient outcomes.
© 2025. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

