Prospective validation study: a non-invasive circulating tumor DNA-based assay for simultaneous early detection of multiple cancers in asymptomatic adults
- PMID: 39948555
- PMCID: PMC11827191
- DOI: 10.1186/s12916-025-03929-y
Prospective validation study: a non-invasive circulating tumor DNA-based assay for simultaneous early detection of multiple cancers in asymptomatic adults
Abstract
Background: Non-invasive multi-cancer early detection (MCED) tests have shown promise in enhancing early cancer detection. However, their clinical utility across diverse populations remains underexplored, limiting their routine implementation. This study aims to validate the clinical utility of a multimodal non-invasive circulating tumor DNA (ctDNA)-based MCED test, SPOT-MAS (Screening for the Presence Of Tumor by DNA Methylation And Size).
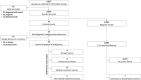
Methods: We conducted a multicenter prospective study, K-DETEK (ClinicalTrials.gov identifier: NCT05227261), involving 9057 asymptomatic individuals aged 40 years or older across 75 major hospitals and one research institute in Vietnam. Participants were followed for 12 months.
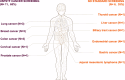
Results: Of the 9024 eligible participants, 43 (0.48%) tested positive for ctDNA. Among these, 17 were confirmed with malignant lesions in various primary organs through standard-of-care (SOC) imaging and biopsy, with 9 cases matching our tissue of origin (TOO) predictions. This resulted in a positive predictive value of 39.53% (95%CI 26.37-54.42) and a TOO accuracy of 52.94% (95%CI 30.96-73.83). Among the 8981 participants (99.52%) who tested negative, 8974 were confirmed cancer-free during a 12-month period after testing, yielding a negative predictive value of 99.92% (95% CI 99.84-99.96). The test demonstrated an overall sensitivity of 70.83% (95%CI 50.83-85.09) and a specificity of 99.71% (95% CI 99.58-99.80) for detecting various cancer types, including those without SOC screening options.
Conclusions: This study presents a prospective validation of a multi-cancer early detection (MCED) test conducted in a lower middle-income country, demonstrating the potential of SPOT-MAS for early cancer detection. Our findings indicate that MCED tests could be valuable additions to national cancer screening programs, particularly in regions where such initiatives are currently limited.
Trial registration: ClinicalTrials.gov ID: NCT05227261. Date of registration: 07/02/2022.
Keywords: Clinical validation; Liquid biopsy; Multicancer early detection; Multimodal analysis; Tissue of origin.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This research received approval from the Ethics Committee of the University of Medicine and Pharmacy, Ho Chi Minh City, Vietnam (approval number: 192/HĐĐĐ-ĐHYD). Each participant provided written informed consent, adhering to the Declaration of Helsinki. Consent for publication: Not applicable. Competing interests: The authors including LST, HNN, HG, MDP, HHN and DSN hold equity in Gene Solutions. NHN, HG, MDP and LST are inventors on the patent application (USPTO 17930705). LHDN, THHN, NMP, BLT, GTHN, DHV, THT, TDN, VTCN, THN, VUT, MPL, TMTT, MNN, TTVV, ANN, TTN, NNTD, HTN, PLD, LAKH, TAN, HTPN, YTL, CTTC, TVP, TTTD, DKT, and HST received salaries from the project funded by the Medical Genetics Institute, Ho Chi Minh City, Vietnam. We confirm that this does not alter our adherence to journal policies on sharing data and materials. VHL, VQB, LHN, NHP, THP, HTN, VST, CVB, VKV, PTNN, HHPD, VDP, VTC, BLT, TTN, HTPN, CTTC, VTN, TLQL, TLAL, TKPD, TTD, CDP, TXN, NTP, BTN, TTTP, HLL, CTT, TXJ, MCL, VBP, QBT, THLT, MTH, TQT, STN, VT, VKT declare that they have no competing interests. VHL, VQB, LHN, NHP, THP, HTN, VST, CVB, VKV, PTNN, HHPD, VDP, VTC, BLT, TTN, HTPN, CTTC, VTN, TLQL, TLAL, TKPD, TTD, CDP, TXN, NTP, BTN, TTTP, HLL, CTT, TXJ, MCL, VBP, QBT, THLT, MTH, TQT, STN, VT, and VKT declare that they have no competing interests.
Figures



References
-
- Hawkes N. Cancer survival data emphasise importance of early diagnosis. BMJ. 2019;364:l408. - PubMed
-
- Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, Donahue KE, Doubeni CA, Krist AH, Kubik M, et al. Screening for colorectal cancer: US preventive services task force recommendation statement. JAMA. 2021;325(19):1965–77. - PubMed
-
- Luo YH, Luo L, Wampfler JA, Wang Y, Liu D, Chen YM, Adjei AA, Midthun DE, Yang P. 5-year overall survival in patients with lung cancer eligible or ineligible for screening according to US Preventive Services Task Force criteria: a prospective, observational cohort study. Lancet Oncol. 2019;20(8):1098–108. - PMC - PubMed
-
- Brill JV. Screening for cancer: the economic, medical, and psychosocial issues. Am J Manag Care. 2020;26(14 Suppl):S300-s306. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

