TREOCAPA: prophylactic treatment of the ductus arteriosus in preterm infants by acetaminophen-statistical analysis plan for the randomized phase III group sequential trial
- PMID: 39948605
- PMCID: PMC11827460
- DOI: 10.1186/s13063-025-08751-8
TREOCAPA: prophylactic treatment of the ductus arteriosus in preterm infants by acetaminophen-statistical analysis plan for the randomized phase III group sequential trial
Abstract
Background: Persistent patency of the ductus arteriosus (PDA) has challenged neonatologists for more than 40 years. Controversies persist about the management of PDA in extremely preterm infants. PDA is associated with morbidities, but no therapeutic strategy has resulted in an improved neonatal outcome. Acetaminophen appears to be a promising alternative with possibly fewer adverse effects. The primary objective is to determine whether a prophylactic pharmacological intervention with acetaminophen may increase the survival without severe morbidity at postmenstrual age of 36 weeks.
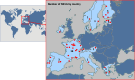
Methods and analysis: TREOCAPA phase III is a randomized, multicenter, double-blind, stratified, placebo-controlled superiority trial, two arms in a 1:1 ratio performed in 43 NICUs of 14 European countries, evaluating whether the intervention increases the survival without severe morbidity by 10%, from 50% in control arm to 60% in treatment arm, until the age of 36 postmenstrual weeks. To detect this difference, 794 patients were required using a group sequential design. Recruitment has been closed, with 803 patients enrolled. Patients eligible for inclusion are preterm infants with a gestational age between 23 and 28 weeks. In the acetaminophen group, 20 mg/kg loading dose within 12 h after birth, followed by 7.5 mg/kg quarter in die (QID) for 5 days, will be administered to the 27-28 weeks gestational age group, and 25 mg/kg loading dose then 10 mg/kg QID will be administered to the 23-26 weeks gestational age group. The severe morbidities include severe bronchopulmonary dysplasia (BPD grade 3) according to NIH consensus, necrotizing enterocolitis (NEC) of Bell's stage II or III, intraventricular hemorrhage (IVH) grade III-IV according to Papile classification, or cystic leukomalacia.
Discussion: Whatever the results, the conclusions of this study should be informative for the neonatal scientific community. The results will either confirm the benefit of treatment in increasing survival without severe morbidity, or indicate a worsening of outcomes with prophylactic acetaminophen treatment, or show no difference in the primary outcome. In the latter case, ultrasonographic assessments of ductus arteriosus status on day 7 may help explain the absence of a difference. This could indicate that acetaminophen is ineffective in promoting ductal closure or that early closure of the ductus arteriosus is inconsequential if, despite more frequent closures, there is no associated improvement in outcomes.
Ethics and dissemination: Ethical approval of the trial has been performed in each of the 14 countries after approval, at the European level, by the Voluntary Harmonization Procedure committee on 04/07/2020. Results will be disseminated through articles in peer-reviewed journals.
Trial registration: European Clinical Trials Database: EudraCT Number: 2019-004297-26.
Clinicaltrials: gov: NCT04459117, registered on July 7, 2020.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
References
-
- Gamble C, Krishan A, Stocken D, Lewis S, Juszczak E, Doré C, et al. Guidelines for the content of statistical analysis plans in clinical trials. JAMA. 2017;318:2337–43. - PubMed
-
- Ancel PY, Goffinet F, EPIPAGE-2 Writing Group, Kuhn P, Langer B, Matis J, et al. Survival and morbidity of preterm children born at 22 through 34 weeks’ gestation in France in 2011: results of the EPIPAGE-2 cohort study. JAMA Pediatr. 2015;169:230–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical