First-in-human evaluation of memory-like NK cells with an IL-15 super-agonist and CTLA-4 blockade in advanced head and neck cancer
- PMID: 39948608
- PMCID: PMC11827236
- DOI: 10.1186/s13045-025-01669-3
First-in-human evaluation of memory-like NK cells with an IL-15 super-agonist and CTLA-4 blockade in advanced head and neck cancer
Abstract
Background: Cytokine induced memory-like natural killer (CIML NK) cells combined with an IL-15 super-agonist (N-803) are a novel modality to treat relapsed/refractory head and neck cancer.
Methods: We report data from a phase I trial of haploidentical CIML NK cells combined with N-803 with or without ipilimumab (IPI) in relapsed/refractory head and neck cancer patients after a median of 6 prior lines of therapy. The trial adhered to a 3 + 3 dose de-escalation design, with primary endpoint being safety. High-resolution immunophenotypic and transcriptional profiling characterized the NK cells and their interacting partners in vivo.
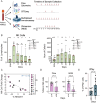
Results: The primary safety endpoint was established, with dose-limiting toxicity in 1/10 patients. A transient disease control rate correlated with donor NK cell expansion, the latter occurring irrespective of IPI. The combination of CIML NK cells with N-803 and IPI was associated with increased early NK cell proliferation, contraction of Treg: Tcon, rapid recovery of recipient CD8+ T cells, and subsequent accelerated rejection of donor NK cells.
Conclusions: CIML NK cells combined with N-803 and ipilimumab to treat head and neck cancer is safe, and associated with a more proliferative NK cell phenotype. However, the combination leads to reduced HLA mismatched NK cell persistence, resulting in an important limitation affecting NK cell combination therapies in clinical trials. These results inform evaluation of CIML NK therapy for advanced malignancies, with considerations for combination with IPI.
Trial registration: NCT04290546.
Keywords: Clinical trial; Head and neck cancer; Interleukin-15; Ipilimumab; NK cells.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: This study was reviewed and approved by the institutional review board of the Dana-Farber Cancer Institute, Boston, MA, USA (Clinicaltrials.gov: NCT04290546). The study was performed in compliance with the provisions of the Declaration of Helsinki and Good Clinical Practice guidelines. Written informed consent was obtained from participants before inclusion in the study. Patients provided written informed consent to participate and to publish under an IRB-approved protocol at the Dana-Farber Cancer Institute where all protocol procedures were performed and data were collected. Role of funding source: The funding source had no role in the design of this study, its execution, analyses, interpretation of the data, or decision to submit the results. Competing interests: RD has received research funding from Ligue Contre le Cancer, Arthur Sachs, Monahan Foundation, Servier Foundation, Philippe Foundation, DCP AP-HP, honoraria from Novartis and Takeda, as well as non-financial support from Kite Pharma / Gilead and Sanofi. MSF receives funding from Calico Life Sciences, Bristol-Myers Squibb, Istari Oncology and served as a consultant for Galvanize Therapeutics. ND and AK are employees of CareDx. JK reports research support from Amgen, Equillium, BMS, Miltenyi Biotec, Regeneron, and Clinigen and consulting income from Amgen, Equillium, and Moderna Therapeutics, and is a scientific advisory board member for Cugene and Therakos. SN reports ad hoc advisory boards for Kite/Gilead, GlaxoSmith Kline, Iovance, A2 Bio and Sobi. JR receives research funding from Kite/Gilead, Novartis and Oncternal Therapuetics and serves in a consulting/advisory role for Garuda Therapeutics, LifeVault Bio, Smart Immune and TriArm Therapeutics. CJW holds equity in BionTech, Inc; and receives research funding from Pharmacyclics. She is a member of the scientific advisory boards of Adventris, Aethon Therapeutics and Repertoire. RJS serves on the board of directors for Be The Match/National Marrow Donor Program; provided consulting for Vor Biopharma, Neovii, CSL Behring, Bluesphere Bio, Cugene, Jasper, Smart Immune; and is on the Data Safety Monitoring board for Juno Therapeutics. GJH receives grant funding to institution from: ACCRF, Actuate, Bicara, BMS, Coherus, Elevar, Gateway for Cancer Research, Genentech, ImmunityBio, KSQ, Kura Oncology, Regeneron, Remix, Replimune, and Secura Bio; and serves in a consulting/advisory role to: Bicara, Coherus, Inhibrx, Kura Oncology, Merck, Naveris, Nextech, OncoSwitch, Regeneron, and Replimune. RR receives funding from CRISPR Therapeutics, Skyline Therapeutics, and is on the advisory board of Glycostem.
Figures




References
-
- Hanna GJ, Patel N, Tedla SG, Baugnon KL, Aiken A, Agrawal N. Personalizing surveillance in Head and Neck Cancer. Am Soc Clin Oncol Educ Book. 2023;43e389718. 10.1200/EDBK_389718. - PubMed
-
- Ferris RL, Licitra L. PD-1 immunotherapy for recurrent or metastatic HNSCC. Lancet. 2019;394(10212):1882–4. 10.1016/S0140-6736(19)32539-5. - PubMed
-
- Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915–28. 10.1016/S0140-6736(19)32591-7. - PubMed
-
- Jung EK, Chu TH, Vo MC, et al. Natural killer cells have a synergistic anti-tumor effect in combination with chemoradiotherapy against head and neck cancer. Cytotherapy. 2022;24(9):905–15. 10.1016/j.jcyt.2022.05.004. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

