miR-200c inhibition and catalase accelerate diabetic wound healing
- PMID: 39948670
- PMCID: PMC11827459
- DOI: 10.1186/s12929-024-01113-7
miR-200c inhibition and catalase accelerate diabetic wound healing
Abstract
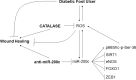
Background: Reactive oxygen species (ROS) are increased in diabetic conditions and play a causal role in diabetic foot ulcers (DFU). We previously showed that ROS up-regulate miR-200c expression, that in turns causes apoptosis, senescence, ROS upregulation and nitric oxide decrease, leading to endothelial disfunction.
Methods: The aim of this study is to dissect miR-200c role in DFU and to explore the potential role of anti-miR-200c and antioxidant catalase (CAT) in promoting wound healing (WH). miR-200c inhibition and CAT treatment were performed either in immortalized keratinocytes (HaCaT) or in primary fibroblasts (FBs) and keratinocytes (KCs) deriving from diabetic patients (pts) undergoing amputations. Primary cells deriving from pts undergoing saphenectomies were used as controls. The miR-200c blockade was performed either via lentiviral particles bearing an anti-miR-200c sequence or locked nucleic acid (LNA) anti-miR-200c oligos. Equine CAT was administered on cell medium. The WH assay was performed in vivo on diabetic (db/db) mice by a topical treatment with CAT and LNA anti-miR-200c on wounds dissolved in a Pluronic gel mixture, administered every three days.
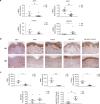
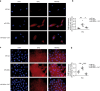
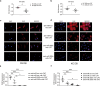
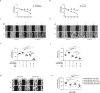
Results: We found that miR-200c levels were increased by different stimuli known to induce ROS, such as ultraviolet radiation (UV), hydrogen peroxide (H2O2), and high glucose in HaCaT. miR-200c was also upregulated in skin biopsies, in FBs and KCs isolated from pts with DFU vs controls. Forced miR-200c expression induced ROS in both FBs and KCs, and CAT reduced it. miR-200c inhibition improved WH in HaCaT, both under basal conditions and after UV and H2O2 treatment, and the simultaneous treatment with CAT accelerated it. miR-200c inhibition accelerated WH in KCs of DFU pts, increasing its protein targets: sirtuin 1 (SIRT1), the transcription factors FOXO1 and ZEB1 and decreasing p66Shc phosphorylation at Ser-36, that is induced by ROS, and the co-treatment with CAT showed synergistic effects in reducing ROS and cytotoxicity. Interestingly, CAT treatment decreased miR-200c expression in FBs and KCs of DFU pts. Topical administration of anti-miR-200c and CAT in a WH model of diabetic mice accelerated closure.
Conclusions: Anti-miR-200c and CAT could be considered a novel treatment for DFU and, possibly, for other types of non-diabetic skin ulcers.
Keywords: Apoptosis; Catalase; Diabetic foot ulcers; MicroRNAs; Reactive oxygen species; Skin.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The human skin biopsies collection was approved by the Ethics Committee of IDI-IRCCS (Prot. N.29/CE/2018 approved on 09/05/2018). Diabetic biopsies were taken from pts with foot ulcers undergoing major amputation from non-lesional area of limb, control skin biopsies were taken from subjects undergoing saphenectomies. All pts provided written informed consent at enrolment. The study was conducted following the Good Clinical Practice guidelines and according to the declaration of Helsinki. Animal experimentation was approved by the animal welfare organism of IDI-IRCCS and authorized by the Animal Committee of Italian Ministry of Health (protocol n. BBA76.2 approval n. 835/2018-PR expired in March 2023). The BMDM extraction was approved by the animal welfare organism of CNR and authorized by the Animal Committee of Italian Ministry of Health (protocol n.6D050.7 approval n.375/2019 PR expired June 2023). Consent for publication: Not applicable. Competing interests: AM declares that the study has been partially funded by Idi Farmaceutici s.r.l. (2018–2019) which commercializes a topical medicine based on equine catalase. DA is employed by Idi Farmaceutici s.r.l., the remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential competing interests.
Figures






References
-
- Henning RJ. Type-2 diabetes mellitus and cardiovascular disease. Future Cardiol. 2018;14(6):491–509. - PubMed
-
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97. - PubMed
-
- Carlomosti F, D’Agostino M, Beji S, Torcinaro A, Rizzi R, Zaccagnini G, et al. Oxidative stress-induced miR-200c disrupts the regulatory loop among SIRT1, FOXO1, and eNOS. Antioxidants Redox Signal. 2017. 10.1089/ars.2016.6643. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

