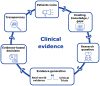
Clinical Evidence 2030
- PMID: 39949283
- PMCID: PMC11924165
- DOI: 10.1002/cpt.3596
Clinical Evidence 2030
Conflict of interest statement
The authors have no competing interests as defined by the American Society for Clinical Pharmacology and Therapeutics, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Figures
References
-
- European Commission . Proposal for a regulation of the European Parliament and of the Council on the European Health Data Space <https://eur‐lex.europa.eu/legal‐content/EN/TXT/?uri=CELEX:52022PC0197> (2022).
-
- European Commission . European Health Union: Commission proposes pharmaceuticals reform for more accessible, affordable and innovative medicines [Press release] <https://ec.europa.eu/commission/presscorner/detail/en/IP_23_1843> (2023).
-
- European Medicines Agency . Engagement framework: European Medicines Agency and patients, consumers and their organisations <https://www.ema.europa.eu/en/partners‐networks/patients‐consumers#framew...> (2022).
-
- Vamvakas, S. , Moseley, J. & Vetter, T. Multistakeholder advice at the European medicines agency: is it still needed? Clin. Pharmacol. Ther. 105, 819–821 (2019). - PubMed
LinkOut - more resources
Full Text Sources