High-frequency oscillations in epileptic and non-epileptic Alzheimer's disease patients and the differential effect of levetiracetam on the oscillations
- PMID: 39949405
- PMCID: PMC11822293
- DOI: 10.1093/braincomms/fcaf041
High-frequency oscillations in epileptic and non-epileptic Alzheimer's disease patients and the differential effect of levetiracetam on the oscillations
Abstract
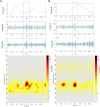
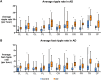
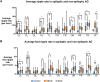
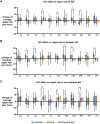
Alzheimer's disease increases the risk of developing epilepsy together with cognitive decline. Early diagnosis or prediction of parameters associated with epileptic activity can greatly help in managing disease outcomes. Network hyperexcitability is a candidate of interest as a neurophysiological biomarker of Alzheimer's disease. High-frequency oscillations are increasingly recognized as potential biomarkers of hyperexcitability and epileptic activity. However, they have not yet been identified in Alzheimer's disease. In this study, we measured high-frequency oscillations via magnetoencephalography recordings in Alzheimer's disease patients with and without epileptic activity, as part of a Phase 2a randomized, double blind clinical trial of the efficacy of levetiracetam to improve cognitive functions in Alzheimer's disease. To measure the high-frequency oscillations, we used 10-min magnetoencephalography recordings (275-channel and sampling rate 1200-4000 Hz) during awake resting periods in participants with Alzheimer's disease and healthy controls. Recordings from 14 Alzheimer's disease participants, with six having non-epileptic Alzheimer's disease (median age: 60.8, 2 M/4 F), eight having sub-clinical epileptic activity (median age: 54.9, 5 M/3 F) and eight as control (median age: 71, 5 M/3 F), were analysed using two software scripts: Delphos and a custom-made script, for detecting high-frequency oscillations. Levetiracetam 125 mg twice-a-day or placebo was administered for 4 weeks in between two magnetoencephalography recordings, and 4 weeks of washout before switching levetiracetam/placebo phases for each participant. High-frequency oscillations were categorized into ripples (80 to 250 Hz) and fast ripples (250 to 500 Hz). At baseline, Alzheimer's disease participants, both epileptic and non-epileptic had higher rate of ripples and fast ripples than controls in several left/right hemispheric sensor regions (P < 0.05). Additionally, compared to epileptic, non-epileptic had higher rate of ripples in left-frontal, left-temporal and cerebral fissure regions and higher rate of fast ripples in left-frontal regions (P < 0.05). In epileptic type, levetiracetam decreased ripples in bilateral-frontal, bilateral-occipital regions and cerebral fissure, whereas in non-epileptic type, levetiracetam increased both ripples and fast ripples in right central and left parietal regions, and ripples in the right parietal region (P < 0.05). Additionally, we found hemisphere asymmetry in epileptic type, with right temporal/occipital having more high-frequency oscillations than their counterpart region. Overall, Alzheimer's disease had a high level of high-frequency oscillations, with higher numbers observed in non-epileptic type. Levetiracetam decreased high-frequency oscillations in epileptic but increased high-frequency oscillations in non-epileptic. Thus, high-frequency oscillations can function as a biomarker of hyperexcitability in Alzheimer's disease and may be more pathological when asymmetric and coinciding with presence of epileptic activity. Levetiracetam has the potential for treating hyperactivity in patients with epileptic Alzheimer's disease.
Keywords: Alzheimer’s disease; epilepsy; high-frequency oscillations; hyperactivity; levetiracetam.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures




Comment in
-
High frequency opportunities for Alzheimer's disease.Brain Commun. 2025 Sep 8;7(5):fcaf328. doi: 10.1093/braincomms/fcaf328. eCollection 2025. Brain Commun. 2025. PMID: 40980403 Free PMC article.
References
-
- Sherzai D, Losey T, Vega S, Sherzai A. Seizures and dementia in the elderly: Nationwide Inpatient Sample 1999–2008. Epilepsy Behav. 2014;36:53–56. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
