Systematic review of innate immune responses against Mycobacterium tuberculosis complex infection in animal models
- PMID: 39949719
- PMCID: PMC11821578
- DOI: 10.3389/fimmu.2024.1467016
Systematic review of innate immune responses against Mycobacterium tuberculosis complex infection in animal models
Abstract
Background: Mycobacterium tuberculosis (Mtb) complex (MTBC) includes ten species that affect mammals and pose a significant global health concern. Upon infection, Mtb induces various stages in the host, including early bacterial elimination, which may or may not involve memory responses. Deciphering the role of innate immune responses during MTBC infection is crucial for understanding disease progression or protection. Over the past decade, there has been growing interest in the innate immune response to Mtb, with new preclinical models emerging.
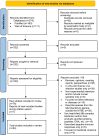
Methods: We conducted a systematic review following PRISMA guidelines, focused on innate immune mediators linked to protection or disease progression in animal models of MTBC infection. We searched two databases: National Library of Medicine and Web of Science. Two researchers independently extracted data based on specific inclusion and exclusion criteria.
Results: Eighty-three articles were reviewed. Results were categorized in four groups: MTBC species, animal models, soluble factors and innate pathways, and other molecules (metabolites and drugs). Mtb and M. bovis were the only species studied. P2X7R receptor's role in disease progression and higher macrophage recruitment were observed differentially after infection with hypervirulent Mtb strains. Mice and non-human primates (NHPs) were the most used mammals, with emerging models like Galleria mellonella and planarians also studied. NHPs provided insights into age-dependent immunity and markers for active tuberculosis (ATB). Key innate immune factors/pathways identified included TNF-α, neutrophil recruitment, ROS/RNS responses, autophagy, inflammasomes, and antimicrobial peptides, with homologous proteins identified in insects. Metabolites like vitamin B5 and prostaglandin E2 were associated with protection. Immunomodulatory drugs targeting autophagy and other mechanisms were studied, exhibiting their potential as therapeutic alternatives.
Conclusion: Simpler, physiologically relevant, and ethically sound models, such as G. mellonella, are needed for studying innate responses in MTBC infection. While insects lack adaptive immunity, they could provide insights into "pure" innate immune responses. The dissection of "pure," "sustained" (later than 7 days post-infection), and trained innate immunity presents additional challenges that require high-resolution temporospatial analytical methods. Identifying early innate immune mediators and targetable pathways in the blood and affected tissues could identify biomarkers for immunization efficiency, disease progression, and potential synergistic therapies for ATB.
Keywords: cytokines; early immunity; in vivo; innate cells; preclinical models; receptors; trained immunity.
Copyright © 2025 Nieto Ramirez, Mehaffy and Dobos.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures



References
-
- World Health Organization . Global Tuberculosis Report 2024. Geneva: (2024).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

