Assessing the clinical meaningfulness of slowing CDR-SB progression with disease-modifying therapies for Alzheimer's disease
- PMID: 39949872
- PMCID: PMC11822626
- DOI: 10.1002/trc2.70033
Assessing the clinical meaningfulness of slowing CDR-SB progression with disease-modifying therapies for Alzheimer's disease
Abstract
Introduction: For many patients and caregivers, a major goal of disease-modifying treatments (DMTs) for Alzheimer's disease (AD) dementia is to extend independence in instrumental and basic activities of daily living (IADLs and BADLs). The goal of this study was to estimate the effect of treatments on the time remaining independent in IADLs and BADLs.
Methods: Participants at the Knight Alzheimer Disease Research Center (Knight ADRC) who met eligibility criteria for recent DMT trials were studied: age ≥60 years at baseline, clinical diagnosis of very mild or mild AD dementia (global Clinical Dementia Rating [CDR] score 0.5 or 1), biomarker confirmation of amyloid pathology, and at least one follow-up CDR assessment within 5 years. For IADLs, a subset of the Functional Assessment Questionnaire (FAQ) was examined that rated the degree of independence in the following: paying bills, driving, remembering medications and appointments, and preparing meals. For BADLs, the Personal Care domain of the CDR was used. Mixed-effects logistic and ordinal regression models were used to examine the relationship between CDR Sum of Boxes (CDR-SB) and the individual functional outcomes and their components. The change in CDR-SB over time was estimated with linear mixed-effects models.
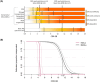
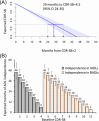
Results: A total of 282 participants were followed for an average of 2.9 years (standard deviation [SD] 1.3 years). For 50% of individuals, loss of independence in IADLs occurred at CDR-SB >4.5 and in BADLs at CDR-SB >11.5. For individuals with a baseline CDR-SB = 2, treatment with lecanemab would extend independence in IADLs for 10 months (95% confidence interval [CI] 4-18 months) and treatment with donanemab in the low/medium tau group would extend independence in IADLs by 13 months (95% CI 6-24 months).
Discussion: Independence in ADLs can be related to CDR-SB and used to demonstrate the effect of AD treatments in extending the time of independent function, a meaningful outcome for patients and their families.
Highlights: We estimated time to loss of independence for people with AD dementiaEstimating time to loss of independence can help with clinical decision-makingDisease-modifying treatments for AD dementia can extend independence.
Keywords: AD dementia; clinical meaningfulness; functional independence.
© 2025 The Author(s). Alzheimer's & Dementia: Translational Research & Clinical Interventions published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors declare no conflicts of interest. Author disclosures are available in the supporting information.
Figures

Update of
-
Assessing the clinical meaningfulness of slowing CDR-SB progression with disease-modifying therapies for Alzheimer disease.medRxiv [Preprint]. 2024 Jul 22:2024.07.16.24310511. doi: 10.1101/2024.07.16.24310511. medRxiv. 2024. Update in: Alzheimers Dement (N Y). 2025 Feb 13;11(1):e70033. doi: 10.1002/trc2.70033. PMID: 39108536 Free PMC article. Updated. Preprint.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
