Cost-effectiveness analysis of the 13-valent pneumococcal conjugate vaccine administered to children under 5 years of age in the Republic of Moldova
- PMID: 39949911
- PMCID: PMC11817585
- DOI: 10.15386/mpr-2674
Cost-effectiveness analysis of the 13-valent pneumococcal conjugate vaccine administered to children under 5 years of age in the Republic of Moldova
Abstract
Background: The Moldovan health authorities introduced the 13 valent pneumococcal conjugate vaccine into the national immunization schedule for children in 2013. This study aimed to evaluate the cost-effectiveness of the pneumococcal conjugate vaccine compared to a no-vaccination strategy in children under 5 Years of age in the Republic of Moldova.
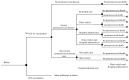
Methods: We used UNIVAC (version 1.7), a static decision model, to evaluate the health and economic outcomes of vaccination in a single-cohort of children under five years. We modeled vaccine introduction over 10 birth cohorts starting in 2013. We assumed a 2+1 (two doses + booster) schedule and a vaccination price of US$ 16.34 per dose. We used locally-specific data for pneumonia incidence, mortality, treatment, and costs. Model outcomes included pneumonia cases, hospitalizations, deaths, disability-adjusted life years, and costs presented in USD. Cost-effectiveness was reported as Incremental Cost Effectiveness Ratio. The Incremental Cost Effectiveness Ratio was calculated to estimate the additional cost to save an additional life year.
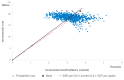
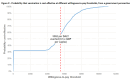
Results: From the governmental health sector the Incremental Cost Effectiveness Ratio was $5939 and from society perspective, $7272, respectively. Withal cost per disability-adjusted life years (DALY) averted was US$ 6311. PCV-13 was projected to prevent 2310 hospitalizations due to pneumococcal disease, including 118 deaths. Vaccination could potentially reduce the highest treatment cost from the payer perspective at $ 4 081 412 for the 13 valent pneumococcal conjugate vaccine.
Conclusion: This study evidenced that cost per DALY averted is US$ 6311, which is between one and three times Gross Domestic Product (GDP) per capita, these findings extrapolate PCV-13 as a cost-effective intervention. Considering the scenario of Republic of Moldova the PCV program is a cost effective intervention and justifies the introduction of PCV into routine immunization schedule throughout the country in order to reduce morbidity and mortality among the under-five-year-old children.
Keywords: cost-effectiveness; immunization programs; pneumococcal; pneumococcal vaccines.
Figures

Similar articles
-
Impact and cost effectiveness of pneumococcal conjugate vaccine in India.Vaccine. 2019 Jan 21;37(4):623-630. doi: 10.1016/j.vaccine.2018.12.004. Epub 2018 Dec 23. Vaccine. 2019. PMID: 30587430
-
Cost-effectiveness of pneumococcal conjugate vaccination in Croatia.Vaccine. 2015 May 7;33 Suppl 1:A209-18. doi: 10.1016/j.vaccine.2014.12.043. Vaccine. 2015. PMID: 25919163
-
Economic evaluation of childhood 7-valent pneumococcal conjugate vaccination in Korea.J Manag Care Pharm. 2010 Jan-Feb;16(1):32-45. doi: 10.18553/jmcp.2010.16.1.32. J Manag Care Pharm. 2010. PMID: 20044845 Free PMC article.
-
Cost-effectiveness analysis of the introduction of the pneumococcal conjugate vaccine (PCV-13) in the Egyptian national immunization program, 2013.Vaccine. 2015 May 7;33 Suppl 1:A182-91. doi: 10.1016/j.vaccine.2014.12.044. Vaccine. 2015. PMID: 25919159
-
Cost effectiveness of pneumococcal conjugate vaccination against acute otitis media in children: a review.Pharmacoeconomics. 2011 Mar;29(3):199-211. doi: 10.2165/11584930-000000000-00000. Pharmacoeconomics. 2011. PMID: 21250759 Review.
References
-
- WHO. Pneumococcal disease. Available from: https://www.who.int/teams/health-product-policy-and-standards/standards-....
-
- World Health Organization. WHO position paper on pneumococcal conjugate vaccines in infants and children under 5 years of age. Weekly Epidemiological Record. 2019;8(Feb 22):85–103. Available from: https://apps.who.int/iris/bitstream/handle/10665/310970/WER9408-85-103.pdf?
-
- Huang L, Wasserman M, Grant L, Farkouh R, Snow V, Arguedas A, et al. Burden of pneumococcal disease due to serotypes covered by the 13-valent and new higher-valent pneumococcal conjugate vaccines in the United States. Vaccine. 2022;40:4700–4708. - PubMed
LinkOut - more resources
Full Text Sources
