Tumor Treating Fields enhance chemotherapy efficacy by increasing cellular drug uptake and retention in mesothelioma cells
- PMID: 39949944
- PMCID: PMC11815374
- DOI: 10.62347/ODWL5634
Tumor Treating Fields enhance chemotherapy efficacy by increasing cellular drug uptake and retention in mesothelioma cells
Abstract
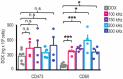
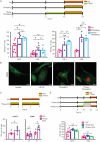
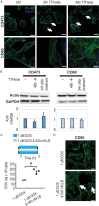
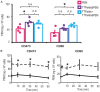
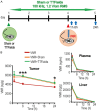
Tumor Treating Fields (TTFields) applied with standard chemotherapy have been approved for the first-line treatment of unresectable pleural mesothelioma (PM), an aggressive malignancy with limited effective therapy options. In this study, we demonstrated that the simultaneous exposure to TTFields and doxorubicin or vinorelbine enhanced treatment efficacy in patient-derived PM cells by increasing intracellular drug concentrations. This was achieved by modulating several genes that encode transport proteins, such as the downregulation of P-glycoprotein (P-gp). Using specific, sensitive and quantitative analytical techniques, we observed a more than 70% increase in intracellular concentrations of doxorubicin and vinorelbine in samples treated with TTFields, and a greater than 50% increase in drug uptake in cells exposed to TTFields and pemetrexed. This result indicates that the increased drug concentration observed in TTFields treated cells is significant not only for drugs that are P-gp substrates but also suggests that TTFields could potentially affect other efflux pumps. However, the co-exposure to the drug and TTFields was critical to increasing intracellular drug levels, highlighting the necessity of concurrent use with drugs to enhance the antiproliferative effects of treatment. The in vitro findings were further corroborated by in vivo pharmacokinetic experiments in mice subcutaneously injected with epithelioid PM tumors. Indeed, a 30% increase in intratumor concentrations was observed when vinorelbine was administered with TTFields. Our findings suggest that TTFields could be a well-tolerated approach for enhancing intratumoral drug levels and potentially achieving a more significant therapeutic impact on PM treatment.
Keywords: Pleural mesothelioma; Tumor Treating Fields (TTFields); cancer therapy; cellular drug uptake; combination treatment; pharmacokinetics.
AJCR Copyright © 2025.
Conflict of interest statement
None.
Figures


References
-
- Baas P, Scherpereel A, Nowak AK, Fujimoto N, Peters S, Tsao AS, Mansfield AS, Popat S, Jahan T, Antonia S, Oulkhouir Y, Bautista Y, Cornelissen R, Greillier L, Grossi F, Kowalski D, Rodríguez-Cid J, Aanur P, Oukessou A, Baudelet C, Zalcman G. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet. 2021;397:375–386. - PubMed
-
- Zhuang W, Liu L, Sun B, Bai H, Wang Z, Duan J, Wan R, Ma Z, Zhong J, Wang J. Evaluation of first-line and salvage therapies for unresectable malignant mesothelioma: a systematic review and network meta-analysis. Crit Rev Oncol Hematol. 2024;198:104372. - PubMed
-
- Zucali PA, De Vincenzo F, Perrino M, Digiacomo N, Cordua N, D’Antonio F, Borea F, Fazio R, Pirozzi A, Santoro A. Advances in drug treatments for mesothelioma. Expert Opin Pharmacother. 2022;23:929–946. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
