Stable luminescent diphenylamine biphenylmethyl radicals with α-type D0 → D1 transition and antiferromagnetic properties
- PMID: 39950058
- PMCID: PMC11815340
- DOI: 10.1039/d4sc08026b
Stable luminescent diphenylamine biphenylmethyl radicals with α-type D0 → D1 transition and antiferromagnetic properties
Abstract
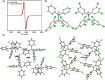
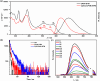
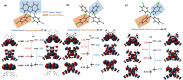
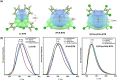
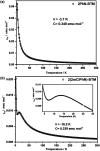
Organic luminescent radicals, with their open-shell electronic structure, exhibit appealing optoelectronic properties and hold promise for a wide range of potential applications. Although a few series of luminescent radicals have been reported, most feature D0 → D1 transitions dominated by HOMOβ-SOMOβ (β-type transitions). In contrast, luminescent radicals that exhibit SOMOα-LUMOα properties (α-type transitions) are much rarer. Here, we designed and synthesized two stable novel diphenylamine biphenylmethyl (BTM) luminescent radicals, both characterized by α-type D0 → D1 transitions, and simultaneously maintained the D-A˙ structure for the first time. Density functional theory (DFT) calculations confirmed that fine-tuning the energy levels of frontier molecular orbitals can facilitate β-type to α-type D0 → D1 transition. Besides, in a 2(2Cl(m)PhN)-BTM crystal we for the first time observed strong antiferromagnetic interactions among luminescent radicals through SQUID measurements. This work offers design insights into luminescent radicals with α-type transition for future development.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
LinkOut - more resources
Full Text Sources

