Epithelial Cell Mechanoresponse to Matrix Viscoelasticity and Confinement Within Micropatterned Viscoelastic Hydrogels
- PMID: 39950757
- PMCID: PMC12079340
- DOI: 10.1002/advs.202408635
Epithelial Cell Mechanoresponse to Matrix Viscoelasticity and Confinement Within Micropatterned Viscoelastic Hydrogels
Abstract
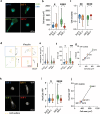
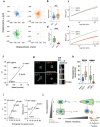
Extracellular matrix (ECM) viscoelasticity has emerged as a potent regulator of physiological and pathological processes, including cancer progression. Spatial confinement within the ECM is also known to influence cell behavior in these contexts. However, the interplay between matrix viscoelasticity and spatial confinement in driving epithelial cell mechanotransduction is not well understood, as it relies on experiments employing purely elastic hydrogels. This work presents an innovative approach to fabricate and micropattern viscoelastic polyacrylamide hydrogels with independently tuneable Young's modulus and stress relaxation, specifically designed to mimic the mechanical properties observed during breast tumor progression, transitioning from a soft dissipative tissue to a stiff elastic one. Using this platform, this work demonstrates that matrix viscoelasticity differentially modulates breast epithelial cell spreading, adhesion, YAP nuclear import and cell migration, depending on the initial stiffness of the matrix. Furthermore, by imposing spatial confinement through micropatterning, this work demonstrates that confinement alters cellular responses to viscoelasticity, including cell spreading, mechanotransduction and migration. These findings establish ECM viscoelasticity as a key regulator of epithelial cell mechanoresponse and highlight the critical role of spatial confinement in soft, dissipative ECMs, which was a previously unexplored aspect.
Keywords: confinement; epithelial cells; extracellular matrix; hydrogels; micropatterning; viscoelasticity.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Guimarães C. F., Gasperini L., Marques A. P., Reis R. L., Nat. Rev. Mater. 2020, 5, 351.
-
- Kechagia J. Z., Ivaska J., Roca‐Cusachs P., Nat. Rev. Mol. Cell Biol. 2019, 20, 457. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
