Performance characteristics of an automated, high-throughput RT-PCR assay for the detection of Candida auris on 3-point and nasal swabs
- PMID: 39950809
- PMCID: PMC11960114
- DOI: 10.1128/spectrum.02114-24
Performance characteristics of an automated, high-throughput RT-PCR assay for the detection of Candida auris on 3-point and nasal swabs
Abstract
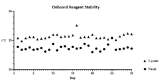
Candida auris is a multidrug-resistant yeast responsible for invasive infections with high mortality rates, primarily spread through prolonged colonization on biotic and abiotic surfaces and traveling. Effective control necessitates comprehensive screening protocols, as recommended by the Centers for Disease Control and Prevention, which endorses a real-time polymerase chain reaction-based assay for C. auris screening. This study evaluates the performance of this assay on the Hologic Panther Fusion System using nasal and 3-point swab specimens (nares/axilla/groin) compared with culture. The assay was assessed for accuracy, sensitivity, specificity, and reproducibility, alongside an evaluation of probe primer reagent (PPR) onboard stability over 30 working days. Analytical sensitivity studies determined limits of detection of 1.95 Log CFU/mL for nasal swabs and 2.18 Log CFU/mL for 3-point swabs, both with >95.0% confidence intervals. The assay demonstrated 100% specificity (n = 25), with no false positives from genetically similar or clinically relevant species, and no significant interference from co-infecting microbes on cycle threshold (CT) values. Both swab types exhibited high intra- and inter-reproducibility, with low coefficients of variation (1.79% and 1.06%, respectively). The assay detected C. auris in 100% of 108 spiked-positive samples across both swab types. Clinical analysis showed 100% concordance with culture for 3-point swabs. Additionally, the nasal swab method showed 96.0% overall agreement, with 86.2% sensitivity and 100.0% specificity. The PPR mixes remained stable over 30 working days, with no significant CT value changes. This study confirms the assay's robust sensitivity, specificity, reproducibility, and accuracy for both nasal and 3-point screening swabs.
Importance: Candida auris is a multidrug-resistant yeast responsible for severe infections with high mortality rates. Rapid and accurate detection is critical for preventing the spread of this pathogen in healthcare settings. This study assesses the performance of an automated real-time PCR screening assay for detecting C. auris using nasal and 3-point swabs. The findings demonstrate the assay's high sensitivity, specificity, and reproducibility, making it a valuable tool for infection control. By providing a reliable and efficient screening method, this assay can significantly enhance efforts to control C. auris outbreaks, ultimately improving patient outcomes and reducing the spread of this dangerous pathogen.
Keywords: Candida auris; PCR; diagnostic; screening.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Validation of a qualitative real-time PCR assay for the detection of Candida auris in hospital inpatient screening.J Clin Microbiol. 2024 Jun 12;62(6):e0015824. doi: 10.1128/jcm.00158-24. Epub 2024 May 1. J Clin Microbiol. 2024. PMID: 38690882 Free PMC article.
-
Performance Evaluation of Five Real-Time PCR Assays for the Detection of Candida auris DNA.Mycoses. 2025 May;68(5):e70065. doi: 10.1111/myc.70065. Mycoses. 2025. PMID: 40317536 Free PMC article.
-
Development and Validation of a Real-Time PCR Assay for Rapid Detection of Candida auris from Surveillance Samples.J Clin Microbiol. 2018 Jan 24;56(2):e01223-17. doi: 10.1128/JCM.01223-17. Print 2018 Feb. J Clin Microbiol. 2018. PMID: 29187562 Free PMC article.
-
Candida auris Diagnostics: Identification and Screening.Clin Lab Med. 2025 Mar;45(1):101-110. doi: 10.1016/j.cll.2024.10.005. Epub 2024 Dec 12. Clin Lab Med. 2025. PMID: 39892930 Review.
-
Diagnosis, management and prevention of Candida auris in hospitals: position statement of the Australasian Society for Infectious Diseases.Intern Med J. 2019 Oct;49(10):1229-1243. doi: 10.1111/imj.14612. Intern Med J. 2019. PMID: 31424595 Review.
References
-
- CDC . 2024. Antibiotic resistance threats in the United States. Atlanta, GA: U.S. Department of Health and Human Services, CDC, 2019
-
- Lyman M, Forsberg K, Reuben J, Dang T, Free R, Seagle EE, Sexton DJ, Soda E, Jones H, Hawkins D, Anderson A, Bassett J, Lockhart SR, Merengwa E, Iyengar P, Jackson BR, Chiller T. 2021. Notes from the field: transmission of pan-resistant and echinocandin-resistant Candida auris in health care facilities—Texas and the District of Columbia, January–April 2021. MMWR Morb Mortal Wkly Rep 70:1022–1023. doi:10.15585/mmwr.mm7029a2 - DOI - PMC - PubMed
-
- Villanueva-Lozano H, Treviño-Rangel R de J, González GM, Ramírez-Elizondo MT, Lara-Medrano R, Aleman-Bocanegra MC, Guajardo-Lara CE, Gaona-Chávez N, Castilleja-Leal F, Torre-Amione G, Martínez-Reséndez MF. 2021. Outbreak of Candida auris infection in a COVID-19 hospital in Mexico. Clin Microbiol Infect 27:813–816. doi:10.1016/j.cmi.2020.12.030 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

