Pyridine indole hybrids as novel potent CYP17A1 inhibitors
- PMID: 39950830
- PMCID: PMC11834790
- DOI: 10.1080/14756366.2025.2463014
Pyridine indole hybrids as novel potent CYP17A1 inhibitors
Abstract
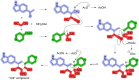
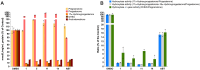
Prostate cancer (PCa) is one of the most prevalent malignancies affecting men worldwide, and androgen deprivation therapy (ADT) is a primary treatment approach. CYP17A1 inhibitors like abiraterone target the steroidogenic pathway to reduce androgen levels, but their clinical efficacy is limited by drug resistance and adverse effects. This study reports the synthesis and evaluation of novel CYP17A1 inhibitors derived from a previously identified hit compound. Several analogs were synthesised, including an unexpected di-cyano derivative, which demonstrated increased potency against CYP17A1 compared to abiraterone. Biological assays revealed that these compounds significantly inhibited CYP17A1 enzymatic activity and altered steroid biosynthesis. Among the newly synthesised inhibitors, compound 11 showed the highest potency (IC50 = 4 nM) and the related compound 14 presented a template for further development. A combined docking and molecular dynamics approach was used to identify the possible target binding modes of the compounds.
Keywords: CYP17A1; enzyme inhibition; inhibitors; prostate cancer.
Conflict of interest statement
The authors report no conflicts of interest.
Figures










References
-
- Siegel RL, Miller KD, Wagle NS, Jemal A.. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. - PubMed
-
- Hughes DL. Review of synthetic routes and crystalline forms of the antiandrogen oncology drugs enzalutamide, apalutamide, and darolutamide. Org Process Res Dev. 2020;24(3):347–362.
-
- Potter GA, Barrie SE, Jarman M, Rowlands MG.. Novel steroidal inhibitors of human cytochrome P45017.alpha.-hydroxylase-C17,20-lyase): potential agents for the treatment of prostatic cancer. J Med Chem. 1995;38(13):2463–2471. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
