Tumor Biomechanics Quantified Using MR Elastography to Predict Response to Neoadjuvant Chemotherapy in Individuals with Breast Cancer
- PMID: 39950962
- PMCID: PMC11966563
- DOI: 10.1148/rycan.240138
Tumor Biomechanics Quantified Using MR Elastography to Predict Response to Neoadjuvant Chemotherapy in Individuals with Breast Cancer
Abstract
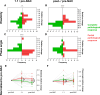
Purpose To evaluate the ability of MR elastography (MRE) to noninvasively quantify tissue biomechanics and determine the added diagnostic value of biomechanics for predicting response throughout neoadjuvant chemotherapy (NAC). Materials and Methods In this prospective study (between September 2020 and August 2023; registration no. NCT03238144), participants with breast cancer scheduled to undergo NAC underwent five MRE scans at different time points alongside clinical dynamic contrast-enhanced MRI (DCE MRI). Regions of interest were drawn over the tumor region for the first two scans, while for the post-NAC scan, the initial pre-NAC tumor footprint was used. Biomechanics, specifically tumor stiffness and phase angle within these regions of interest, were quantified as well as the corresponding ratios relative to before NAC (tumor-stiffness ratio and phase-angle ratio, respectively). Postsurgical pathologic analysis was used to determine complete and partial responders. Furthermore, a repeatability analysis was performed for 18 participants. Results Datasets of 41 female participants (mean age, 47 years ± 12.5 [SD]) were included in this analysis. The tumor-stiffness ratio following NAC decreased significantly for complete responders and increased for partial responders (0.76 ± 0.16 and 1.14 ± 0.24, respectively; P < .001). The phase-angle ratio after the first cycle of the first NAC regimen compared with before NAC predicted pathologic response (1.23 ± 0.31 vs 0.91 ± 0.34; P < .001). Combining the tumor stiffness ratio with DCE MRI improved specificity compared with DCE MRI alone (96% vs 44%) while maintaining the high sensitivity of DCE MRI (94%). Repeatability analysis showed excellent agreement for elasticity (repeatability coefficient, 8.3%) and phase angle (repeatability coefficient, 5%). Conclusion MRE-derived phase-angle ratio and tumor stiffness ratio were associated with pathologic complete response in participants with breast cancer undergoing NAC, and a combined DCE MRI plus MRE approach significantly enhanced specificity for identification of complete responders after NAC, while maintaining high sensitivity. Keywords: Breast Cancer, MR Elastography, Neoadjuvant Chemotherapy, Dynamic Contrast-enhanced MRI Supplemental material is available for this article. Clinical trials registration no. NCT03238144 Published under a CC BY 4.0 license.
Keywords: Breast Cancer; Dynamic Contrast-enhanced MRI; MR Elastography; Neoadjuvant Chemotherapy.
Conflict of interest statement
Figures

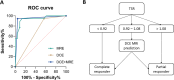
![Flowchart and study pathway. (A) Flowchart of participant inclusion.
Ultimately, datasets from 41 participants who fulfilled all necessary
criteria (ie, all scans performed throughout neoadjuvant chemotherapy [NAC],
all scans with sufficient quality, and post-NAC histopathologic analyses
available) were included in this analysis. (B) Participants received three
or four cycles of the first regimen and three or four cycles of the second
regimen prior to post-NAC surgical intervention. In total, five MRI and MR
elastography (MRE) sessions were interlaced with the NAC regimen.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/738b/11966563/653fd90ca5df/rycan.240138.fig1.gif)



References
-
- Lifetime risk estimates calculated by the Cancer Intelligence Team at Cancer Research UK . https://www.cancerresearchuk.org/health-professional/cancer-statistics/s.... Published 2023. Updated December 14, 2023. Accessed 2024 .
-
- Simon A , Robb K . Cancer: breast . In: Ayers S , Baum A , McManus C , et al. , eds. Cambridge Handbook of Psychology, Health and Medicine . 2nd ed. Cambridge University Press; , 2022. ; 577 – 580 .
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

