The effect of therapeutic plasma exchange on the inflammatory response in septic shock: a secondary analysis of the EXCHANGE-1 trial
- PMID: 39951217
- PMCID: PMC11828778
- DOI: 10.1186/s40635-025-00725-z
The effect of therapeutic plasma exchange on the inflammatory response in septic shock: a secondary analysis of the EXCHANGE-1 trial
Abstract
Background: Sepsis and septic shock, defined by a profound immune dysregulation, are among the leading causes of death in the intensive care unit (ICU). Despite advances in understanding the underlying pathophysiology, evidence for specific immunomodulatory treatment does not exist to date. Therapeutic plasma exchange (TPE) represents an adjunctive treatment approach to rebalance immune homeostasis. In the EXCHANGE-1 trial, we recently demonstrated a rapid hemodynamic improvement, possibly caused by the removal of harmful mediators and the replacement of protective plasma proteins. The aim of this secondary analysis is to further characterize the underlying immunomodulatory effects and to identify biomarkers that may predict treatment response.
Methods: This secondary analysis included patients in early septic shock (< 24 h duration) and a norepinephrine (NE) dose of ≥ 0.4 μg/kg/min. Patients were randomized 1:1 to receive standard of care (SOC) or SOC + one single TPE and plasma samples were collected before and after TPE. Within-group and between group effects of circulating levels of acute-phase proteins [CRP and Pentraxin3 (PTX3)], inflammatory mediators (IL-4, IL-6, IL-8, IL-10, TNF-α, IL-2Rα/CD25) and damage-associated molecular pattern (DAMP) [cell-free DNA (cfDNA)] were analyzed via paired t test or Wilcoxon signed-rank test and a mixed-effects model. Multivariate mixed-effects modeling of NE and lactate reduction was performed to investigate if cfDNA could be associated with treatment response to TPE.
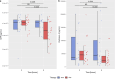
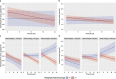
Results: TPE led to a significant reduction in circulating acute-phase protein levels (CRP p = 0.00976, PTX3 p = 0.0001). Pro-inflammatory cytokines, such as circulating TNF-α-, IL-6- und IL-8-levels, were significantly reduced in both groups with no significant difference between treatment groups except for IL-2Rα/CD25 (p ≤ 0.0001). In a multivariate mixed-effects model, rising cfDNA levels over the first 6 h indicated refractoriness to SOC treatment regarding NE (p = 0.004) and lactate (p = 0.001), whereas those receiving TPE demonstrated sustained reductions in both parameters.
Conclusions: In this secondary analysis of the EXCHANGE-1 trial adjunctive TPE is associated with the reduction of acute-phase proteins and IL-2Rα/CD25, however not with the reduction of pro-inflammatory cytokines. This phenomenon could contribute to the observed enhancement in hemodynamics among patients with septic shock. Furthermore, TPE may be particularly beneficial for patients with septic shock who exhibit rising levels of cfDNA.
Keywords: Blood purification; Cell-free nucleic acids; Damage-associated molecular pattern molecules; Extracorporeal treatment; Fresh frozen plasma; Immune response; Plasmapheresis; Precision medicine; Sepsis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The trial was conducted according to the principles of the Declaration of Helsinki and was approved by the Institutional Review Board of the Hannover Medical School (No. 2786–2015 and No. 8852_MPG_23b_2020) and the University Hospital Bonn (No. 024/20). Written informed consent was provided by participants or their legal representatives prior to enrollment. The study was registered at clincaltrials.gov (Identifier: NCT04231994). Consent for publication: Not applicable. Competing interests: Sascha David is supported by an unrestricted research Grant from Cytosorbents for an RCT to test CytoSorb in CRS (CYTORELEASE-Ex). The other authors declare that they have no competing interests.
Figures


References
-
- Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R et al (2017) Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 43(3):304–377 - PubMed
-
- Steinhagen F, Schmidt SV, Schewe JC, Peukert K, Klinman DM, Bode C (2020) Immunotherapy in sepsis—brake or accelerate? Pharmacol Ther 208:107476 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

