Automated Insulin Delivery in Adults With Type 2 Diabetes: A Nonrandomized Clinical Trial
- PMID: 39951268
- PMCID: PMC11829226
- DOI: 10.1001/jamanetworkopen.2024.59348
Automated Insulin Delivery in Adults With Type 2 Diabetes: A Nonrandomized Clinical Trial
Abstract
Importance: There is a need for additional treatment options for people with type 2 diabetes treated with insulin. Given the limited data on the use of automated insulin delivery (AID) systems in type 2 diabetes, studies evaluating their safety and efficacy are important.
Objective: To evaluate the association of AID with hemoglobin A1c (HbA1c) levels in a diverse cohort of adults with type 2 diabetes.
Design, setting, and participants: This single-arm prospective trial was conducted at 21 clinical centers in the United States among individuals aged 18 to 75 years with type 2 diabetes who had been using insulin for at least 3 months prior to screening. Participants with AID system use were excluded. The study started with a 14-day standard therapy phase, followed by 13 weeks of treatment with the investigational device. The first participant was enrolled April 11, 2023, and the last participant follow-up visit was February 29, 2024.
Intervention: Participants used the Omnipod 5 AID System for 13 weeks following the 14-day standard therapy phase.
Main outcomes and measures: Primary outcome was change in HbA1c level at 13 weeks, tested sequentially for noninferiority (0.3% margin) and superiority, compared with baseline.
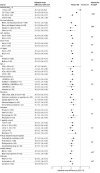
Results: Among 305 participants (mean [SD] age, 57 [11] years; 175 [57%] female; 72 [24%] Black, 66 [22%] Hispanic or Latino, and 153 [50%] White), 289 (95%) completed the trial. At baseline, 223 (73%) were using multiple daily injections, 63 (21%) were using basal insulin without bolus, 17 (6%) were using an insulin pump, 188 (62%) were using continuous glucose monitoring, 168 (55%) were using glucagon-like peptide-1 receptor agonists (GLP-1RAs), and 134 (44%) were using sodium-glucose transport protein 2 inhibitors (SGLT-2is). Following AID use, HbA1c levels decreased from a mean (SD) of 8.2% (1.3) at baseline to 7.4% (0.9) at 13 weeks (mean difference, -0.8 [95% CI, -1.0 to -0.7] percentage points; P < .001 for noninferiority and superiority). Improvement was seen across various subgroups (age, sex, race and ethnicity, insurance), and notably with or without use of GLP-1RAs or SGLT-2is and regardless of pretrial mealtime insulin regimen. Time in target glucose range (70-180 mg/dL) increased from a mean (SD) of 45% (25) to 66% (17) (mean difference, 20 [95% CI, 18 to 22] percentage points; P < .001). Percentage of time in hypoglycemic ranges of less than 54 mg/dL and less than 70 mg/dL was noninferior compared with standard therapy. There was 1 episode of severe hypoglycemia and none of diabetic ketoacidosis or hyperosmolar hyperglycemic syndrome.
Conclusions and relevance: In this nonrandomized clinical trial, HbA1c levels were lower in a diverse cohort of adults with type 2 diabetes following AID initiation, suggesting that AID may be a beneficial and safe option for people with type 2 diabetes using insulin.
Trial registration: ClinicalTrials.gov Identifier: NCT05815342.
Conflict of interest statement
Figures
References
-
- US Centers for Disease Control and Prevention. National diabetes statistics report. May 15, 2024. Accessed January 6, 2025. https://www.cdc.gov/diabetes/php/data-research/index.html
-
- Sherr JL, Bode BW, Forlenza GP, et al. ; Omnipod 5 in Preschoolers Study Group . Safety and glycemic outcomes with a tubeless automated insulin delivery system in very young children with type 1 diabetes: a single-arm multicenter clinical trial. Diabetes Care. 2022;45(8):1907-1910. doi:10.2337/dc21-2359 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

