Evaluating elexacaftor/tezacaftor/ivacaftor (ETI; Trikafta™) for treatment of patients with non-cystic fibrosis bronchiectasis (NCFBE): A clinical study protocol
- PMID: 39951444
- PMCID: PMC11828409
- DOI: 10.1371/journal.pone.0316721
Evaluating elexacaftor/tezacaftor/ivacaftor (ETI; Trikafta™) for treatment of patients with non-cystic fibrosis bronchiectasis (NCFBE): A clinical study protocol
Abstract
Background: Non-cystic fibrosis bronchiectasis (NCFBE) is a disease that exhibits dilatation of airways, airflow obstruction, persistent cough, excessive sputum production, and refractory respiratory infections. NCFBE exhibits clinical and pathological manifestations similar to key features of cystic fibrosis (CF) lung disease. In CF, pathogenesis results from dysfunction of the cystic fibrosis transmembrane conductance regulator (CFTR), and diagnosis is made by demonstrating elevated sweat chloride concentrations (typically ≥60 mEq/L), two CFTR mutations known to be causal, multi-organ tissue injury, or combination(s) of these findings.
Objective: Based on a considerable body of evidence, we believe many patients with NCFBE have disease likely to benefit from drugs such as elexacaftor/tezacaftor/ivacaftor (ETI) that activate CFTR-dependent ion transport. ETI is currently prescribed solely for treatment of CF and has not been adequately tested or proposed for patients with NCFBE, many of whom exhibit decreased CFTR function. Accordingly, we are conducting a clinical trial of ETI in subjects carrying a diagnosis of NCFBE.
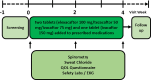
Methods: Participants will exhibit one disease-causing CFTR mutation and/or sweat chloride measurements of 30-59 mEq/L. Cutaneous punch biopsy or blood samples will be obtained for iPS cell differentiation into airway epithelial monolayers-which will then be tested for response to ETI. Each patient will be given CFTR modulator treatment for approximately four weeks, with monitoring of clinical endpoints that include FEV1 (forced expiratory volume in one second), sweat chloride, quality of life questionnaire, and weight. The study will evaluate response of patients with NCFBE to ETI, and test usefulness of iPSC-derived airway epithelial monolayers as a novel in vitro technology for predicting clinical benefit.
Trial registration: This trial is registered at clinicaltrials.gov (Identifier: NCT05743946. Date: 02/23/2023).
Copyright: © 2025 Swenson et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have read the journal’s policy and have the following competing interests: Eric J. Sorscher is a member of the Board of Trustees for the Cystic Fibrosis Foundation. His laboratory develops drugs for the treatment of airway diseases. Emory University has filed a patent application (PCT/US23/60828; Treatment of Bronchiectasis) listing Sorscher, William R. Hunt, and Arlene A. Stecenko as inventors. Authors of this manuscript are also listed as inventors on previous patents: (1.) CFTR activator compounds intended primarily for treatment of cystic fibrosis [CM, JSH, AR, EJS; PCT/US2021/043956: Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Modulators, Pharmaceutical Compositions, and Uses Thereof] and (2.) derivation of airway basal cells from iPSCs [BRD, SS, CB; US 11,401,510 B2: Generation of Airway Basal Stem Cells from Human Pluripotent Stem Cells]. All other authors have declared no competing interests exist. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical