Characterizing the heterogeneity of Castleman disease and oligocentric subtype: findings from the ACCELERATE registry
- PMID: 39951615
- PMCID: PMC12018988
- DOI: 10.1182/bloodadvances.2024014391
Characterizing the heterogeneity of Castleman disease and oligocentric subtype: findings from the ACCELERATE registry
Abstract
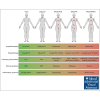
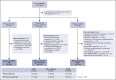
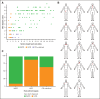
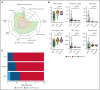
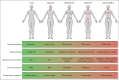
Castleman disease (CD) describes a group of rare lymphoproliferative disorders that exhibit a wide range of symptomatology and degree of lymphadenopathy, particularly across the 2 forms of CD with unknown etiology, unicentric CD (UCD), and human herpesvirus-8-negative/idiopathic multicentric CD (iMCD). Whereas UCD cases typically present with localized lymphadenopathy and mild symptoms, iMCD involves multicentric lymphadenopathy and cytokine storm-driven symptoms with 3 recognized clinical phenotypes. Increasingly, there are anecdotal reports of cases that do not fit into this framework, but these cases have not been systematically described. Herein, we use the ACCELERATE natural history registry to characterize the spectrum of CD based on disease features, symptomatology, and severity. Our results characterize a cohort of 179 CD cases, which were reviewed and confirmed by an expert panel of clinicians and hematopathologists. We show that patients with CD present on a continuous spectrum of clinical phenotypes, and we describe oligocentric CD (OligoCD), an intermediate phenotype that does not fit the criteria for UCD or iMCD. These cases tend to have "oligocentric" lymphadenopathy (median [interquartile range] regions of lymphadenopathy, 3.0 [2.0-4.0]) in a regional pattern and exhibit a mild clinical phenotype that is more similar to UCD than iMCD. We also show that patients with OligoCD are inconsistently categorized as UCD vs iMCD, highlighting the need for this characterization. Future data collected through ACCELERATE may further elucidate the natural history and risk profile of these patients.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: J.D.B. is supported by a Doris Duke Foundation Physician Scientist Fellowship and American Society of Transplantation and Cellular Therapy New Investigator Award; and has consulted for Recordati Rare Diseases and EUSA Pharma. D.A.T. is a cofounder of Quantitative Radiology Solutions LLC. M.J.L. reports consulting fees from Secura Bio, EUSA Pharma, and Kyowa Kirin, and travel support from Secura Bio. C.C. is a paid consultant and speaker for Recordati Rare Diseases, and receives research funding from Johnson & Johnson and Immunity Bio. A.D. has served on the advisory board and independent review committees for Janssen Pharmaceuticals; has received research funding from Alnylam, Pfizer, Takeda, and Bristol Myers Squibb; and serves on the scientific advisory board for AbbVie and HaemaLogiX. R.K. has received research funding from Boehringer Ingelheim, Debiopharm, Foundation Medicine, Genentech, Grifols, Guardant, Incyte, Konica Minolta, MedImmune, Merck Serono, Omniseq, Pfizer, Sequenom, Takeda, TopAlliance, and the National Cancer Institute; has received consultant and/or speaker fees and/or advisory board/consultant fees from Actuate Therapeutics, AstraZeneca, Bicara Therapeutics Inc, Biological Dynamics, Caris, Datar Cancer Genetics, Daiichi, Eisai, EOM Pharmaceuticals, Iylon, LabCorp, Merck, NeoGenomics, Neomed, Pfizer, Precirix, Prosperdtx, Regeneron, Roche, TD2/Volastra, Turning Point Therapeutics, and X-Biotech; has an equity interest in CureMatch Inc; serves on the board of CureMatch and CureMetrix; and is a cofounder of CureMatch. S.M. reports advisory board participation for Celgene/Acceleron, Bristol Myers Squibb, Novartis, Blueprint Medicines, Genentech, EUSA/Recordati, and AbbVie; reports honoraria from Aplastic Anemia and MDS International Foundation, Celgene (now Bristol Myers Squibb), Bristol Myers Squibb, McGraw Hill Hematology Oncology Board Review, Partnership for Health Analytic Research, LLC, and EUSA/Recordati; reports consultancy for BioPharm, Celgene, Novartis, Bristol Myers Squibb, EUSA/Recordati; and received research funding (institution funding) from Bristol Myers Squibb (formerly Celgene), Novartis, and Jazz Pharmaceuticals. S.N. reports research funding from Caribou biosciences, ONO therapeutics, Astex, Atara, LOXO/Lilly, Pharmacyclics, Genentech/Roche, and Seattle Genetics; reports advisory board membership from Genmab, ADC Therapeutics, Genentech, and Acrotech; and reports data safety monitoring board membership with Merck. J.-T.N. received research grants not related to this manuscript from Gilead and EUSA Pharma/Recordati Rare Diseases; and received honoraria not related to this manuscript from AstraZeneca, Blueprint Medicines, EUSA Pharma/Recordati Rare Diseases, Novartis, and Roche. A.N. received research funding from Pharmacyclics/AbbVie, Kite/Gilead, and Cornerstone; received consulting fees from Janssen, MorphoSys, Cornerstone, Epizyme, EUSA, TG therapeutics, ADC Therapeutics, and AstraZeneca; and received honoraria from Pharmacyclics/AbbVie. M.S. reports speakers bureau and consultancy fees from Regeneron. R.S.M.W. has received grants/research support from AbbVie, Acerta, Alexion, Amgen, Apellis, Astella, AstraZeneca, Bayer, Bristol Myers Squibb, Boehringer-Ingelheim, Celgene, Daiichi-Sankyo, GSK, Janssen, Kartos, MorphoSys, MSD, Novartis, Pfizer, Regeneron, and Roche; and consultant and/or speaker fees and/or advisory board/consultant fees from Alexion, Amgen, Astella, AstraZeneca, Bayer, Bristol Myers Squibb, Boehringer-Ingelheim, Daiichi-Sankyo, GlaxoSmithKline, Novartis, Pfizer, and Roche. M.S.L. reports research funding and advisory board and speakers bureau fees from Thermo Fisher Scientific. G.S. reports speakers bureau fees from Recordati Rare Diseases. F.v.R. has received consulting fees from EUSA Pharma, GlaxoSmithKline, Janssen, Karyopharm, and Takeda; and has received research funding from Janssen Pharmaceuticals and Bristol Myers Squibb. D.C.F. receives funding from the National Heart, Lung, and Blood Institute (R01HL141408; 2018 to present); has consulted for and received research funding from Recordati Rare Diseases and EUSA Pharma; was provided study drug for a clinical trial of a Pfizer product (sirolimus); and has provisional patent applications filed by the University of Pennsylvania (provisional patent applications: 63/113,045; 62/989,437). The remaining authors declare no competing financial interests.
Figures
References
-
- Castleman B, Iverson L, Menendez V. Localized mediastinal lymphnode hyperplasia resembling thymoma. Cancer. 1956;9(4):822–830. - PubMed
-
- Dispenzieri A, Fajgenbaum DC. Overview of Castleman disease. Blood. 2020;135(16):1353–1364. - PubMed
-
- Nishimura Y, Fajgenbaum DC, Pierson SK, et al. Validated international definition of the thrombocytopenia, anasarca, fever, reticulin fibrosis, renal insufficiency, and organomegaly clinical subtype (TAFRO) of idiopathic multicentric Castleman disease. Am J Hematol. 2021;96(10):1241–1252. - PMC - PubMed
-
- Iwaki N, Fajgenbaum DC, Nabel CS, et al. Clinicopathologic analysis of TAFRO syndrome demonstrates a distinct subtype of HHV-8-negative multicentric Castleman disease. Am J Hematol. 2016;91(2):220–226. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

