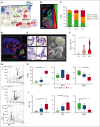
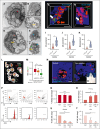
In vitro studies of human erythropoiesis using a 3D silk-based bone marrow model that generates erythroblastic islands
- PMID: 39951616
- PMCID: PMC12083923
- DOI: 10.1182/bloodadvances.2024014905
In vitro studies of human erythropoiesis using a 3D silk-based bone marrow model that generates erythroblastic islands
Abstract
The pursuit of ex vivo erythrocyte generation has led to the development of various culture systems that simulate the bone marrow microenvironment. However, these models often fail to fully replicate the hematopoietic niche's complex dynamics. In our research, we use a comprehensive strategy that emphasizes physiological red blood cell (RBC) differentiation using a minimal cytokine regimen. A key innovation in our approach is the integration of a 3-dimensional (3D) silk-based scaffold engineered to mimic both the physical and chemical properties of human bone marrow. This scaffold facilitates critical macrophage-RBC interactions and incorporates fibronectin functionalization to support the formation of erythroblastic island (EBI)-like niches. We observed diverse stages of erythroblast maturation within these niches, driven by the activation of autophagy, which promotes organelle clearance and membrane remodeling. This process leads to reduced surface integrin expression and significantly enhances RBC enucleation. Using a specialized bioreactor chamber, millions of RBCs can be detached from the EBIs and collected in transfusion bags via dynamic perfusion. Inhibition of autophagy through pharmacological agents or α4 integrin blockade disrupted EBI formation, preventing cells from completing their final morphological transformations, having them trapped in the erythroblast stage. Our findings underscore the importance of the bone marrow niche in maintaining the structural integrity of EBIs and highlight the critical role of autophagy in facilitating organelle clearance during RBC maturation. RNA sequencing analysis further confirmed that these processes are uniquely supported by the 3D silk scaffold, which is essential for enhancing RBC production ex vivo.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures