Circulating extracellular vesicles as potential biomarkers and mediators of acute respiratory distress syndrome in sepsis
- PMID: 39953195
- PMCID: PMC11829037
- DOI: 10.1038/s41598-025-89783-7
Circulating extracellular vesicles as potential biomarkers and mediators of acute respiratory distress syndrome in sepsis
Abstract
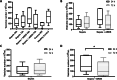
The early sequence of respiratory failure events after the onset of sepsis is still unknown. We hypothesize that the lung should signal through circulating extracellular vesicles (EVs) when it is affected by a systemic inflammatory response. Blood samples were obtained from septic patients with (n = 5) and without acute respiratory distress syndrome (ARDS) (n = 13) at 24 h of intensive care unit admission and 3 days later at Sírio-Libanês Hospital. Pulmonary-originated sepsis was not considered. The characteristics of the plasma-isolated EVs were compatible with exosomes. 48 miRNAs were evaluated by real-time PCR. Comparing all samples from patients with sepsis + ARDS to sepsis only, 9 miRNAs are transported in smaller amounts: miR-766 (-35.7, p = 0.002), miR-127 (-23.8, p = 0.001), miR-340 (-13.5, p = 0.006), miR-29b (-12.8, p = 0.001), miR-744 (-7.1, p = 0.05), miR-618 (-4.0, p = 0.02), miR-598 (-3.8, p = 0.035), miR-1260 (-2.5, p = 0.035); and miR-885-5p is expressed at higher levels (9.5; p = 0.028). In paired samples, the set of altered miRNAs is generally different (p < 0.05) between sepsis + ARDS (miR-1183,-1267,-1290,-17,-192,-199a-3p,-25,-485-3p,-518d,-720) or sepsis only (miR-148a,-193a-5p,-199a-3p,-222,-25,-340,744). Bioinformatic analysis showed that when sepsis is associated with ARDS, those differentially expressed miRNAs potentially target messenger RNAs from the Glycoprotein VI/GP6 signaling pathway. Circulating EV-miRNA cargo could be potential biomarkers of lung inflammation during sepsis in patients requiring mechanical ventilation.
Keywords: ARDS; Exosomes; Extracellular microvesicles; MiRNA; Sepsis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Support: This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo - FAPESP (grant number 2015/20703-1).
Figures




References
-
- Amato, M. B. et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl. J. Med.338, 347–354. 10.1056/NEJM199802053380602 (1998). - PubMed
-
- Sharma, S. & Kumar, A. Septic shock, multiple organ failure, and acute respiratory distress syndrome. Curr. Opin. Pulm Med.9, 199–209. 10.1097/00063198-200305000-00008 (2003). - PubMed
-
- ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin definition. JAMA307, 2526–2533. 10.1001/jama.2012.5669 (2012). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

