Artificial intelligence for individualized treatment of persistent atrial fibrillation: a randomized controlled trial
- PMID: 39953289
- PMCID: PMC12003177
- DOI: 10.1038/s41591-025-03517-w
Artificial intelligence for individualized treatment of persistent atrial fibrillation: a randomized controlled trial
Abstract
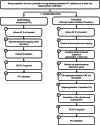
Although pulmonary vein isolation (PVI) has become the cornerstone ablation procedure for atrial fibrillation (AF), the optimal ablation procedure for persistent and long-standing persistent AF remains elusive. Targeting spatio-temporal electrogram dispersion in a tailored procedure has been suggested as a potentially beneficial alternative to a conventional PVI-only procedure. In this multicenter, randomized, controlled, double-blind, superiority trial, patients with drug-refractory persistent AF were randomly assigned to a tailored ablation procedure targeting areas of spatio-temporal dispersion, as detected by an artificial intelligence (AI) algorithm, in addition to PVI (tailored arm, n = 187, 23% women) or to a conventional PVI-only procedure (anatomical arm, n = 183, 19% women). The primary efficacy endpoint was freedom from documented AF with or without antiarrhythmic drugs at 12 months after a single ablation procedure. Secondary endpoints included freedom from any atrial arrhythmic events, and the secondary composite safety endpoint consisted of death, cerebrovascular events, or treatment-related serious adverse events. One year post-procedure, the trial met its primary efficacy endpoint, which was achieved in 88% of patients in the tailored arm compared with 70% of patients in the anatomical arm (log-rank P < 0.0001 for superiority). However, no significant difference between arms was observed for the freedom from any atrial arrhythmia endpoint after one ablation. The safety endpoint did not differ between arms, with procedure and ablation times being twice as long in the tailored arm. These results show that AI-guided ablation of spatio-temporal dispersion areas in addition to PVI is superior to PVI alone in eliminating AF at 1-year follow-up in patients with persistent and long-standing persistent AF. Ablation of subsequent organized atrial tachycardias may be needed to maintain sinus rhythm long term. ClinicalTrials.gov identifier: NCT04702451 .
© 2025. The Author(s).
Conflict of interest statement
Competing interests: I.D. has received speaker honoraria and travel grants from Abbott Medical, Biosense Webster, Boston Scientific, Daiichi Sankyo, Bristol Myer Squibbs, and Volta Medical. J.-P.A. has served as a consultant for Abbott Medical, Medtronic, and Volta Medical. S.B. has received speaker and consultancy honoraria from Biosense Webster, Medtronic, Biotronik, Impulse Dynamics, Pfizer, and Volta Medical. E.G. has served as a consultant for Abbott Medical. S.E.M. has received honoraria from Volta Medical. J.H. has served as a consultant for Abbott Medical and has received honoraria for participation in advisory board from Medtronic. S.O. has served as a consultant for Volta Medical, Biosense Webster, Boston Scientific, GE Healthcare, AtriCure, and PaceMate, has received research grant from Boston Scientific, and is a shareholder of HRCRS/3PH and PaceMate. F.B. has served as a consultant for Biosense Webster and Volta Medical. T.D.P. has served as a consultant for Boston Scientific and has received research grants from Biosense Webster. C.D.C. has received speaker honoraria and travel grants from Biosense Webster, Abbott Medical, and Boston Scientific. S.G. has received honoraria for participation in advisory board and equity from Volta Medical. A.V. has received research grants from Biosense Webster, Medtronic, and Bayer, and has served in advisory boards at Biosense Webster, Medtronic, Volta Medical, Medlumics, Abbott Medical, and Adagio. J.D.H. has served as a consultant for Medtronic, Abbott Medical, and Volta Medical. T.M.D. is a co-founder, shareholder, and CEO of Volta Medical. P.M., A.A., and D.G. are employees of Volta Medical. The other authors declare no competing interests.
Figures






References
-
- Sheikh, A. et al. Trends in hospitalization for atrial fibrillation: epidemiology, cost, and implications for the future. Prog. Cardiovasc. Dis.58, 105–116 (2015). - PubMed
-
- Hindricks, G. et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J.42, 373–498 (2021). - PubMed
-
- Chiang, C.-E. et al. Distribution and risk profile of paroxysmal, persistent, and permanent atrial fibrillation in routine clinical practice: insight from the real-life global survey evaluating patients with atrial fibrillation international registry. Circ: Arrhythm. Electrophysiol.5, 632–639 (2012). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

