Nitroglycerin-responsive gene switch for the on-demand production of therapeutic proteins
- PMID: 39953326
- PMCID: PMC12270905
- DOI: 10.1038/s41551-025-01350-7
Nitroglycerin-responsive gene switch for the on-demand production of therapeutic proteins
Abstract
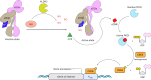
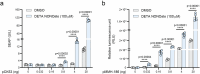
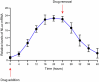
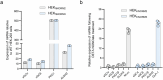
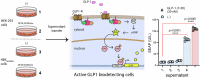
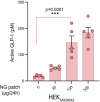
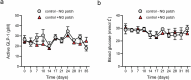
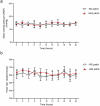
Gene therapies and cell therapies require precise, reversible and patient-friendly control over the production of therapeutic proteins. Here we present a fully human nitric-oxide-responsive gene-regulation system for the on-demand and localized release of therapeutic proteins through clinically licensed nitroglycerin patches. Designed for simplicity and robust human compatibility, the system incorporates human mitochondrial aldehyde dehydrogenase for converting nitroglycerin into nitric oxide, which then activates soluble guanylate cyclase to produce cyclic guanosine monophosphate, followed by protein kinase G to amplify the signal and to trigger target gene expression. In a proof-of-concept study, human cells expressing the nitroglycerin-responsive system were encapsulated and implanted subcutaneously in obese mice with type 2 diabetes. Transdermal nitroglycerin patches applied over the implant enabled the controlled and reversible production of glucagon-like peptide-1 throughout the 35-day experimental period, effectively restoring blood glucose levels in these mice without affecting heart rate or blood pressure. The approach may facilitate the development of safe, convenient and responsive implantable devices for the sustained delivery of biopharmaceuticals for the management of chronic diseases.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





References
-
- Muller, M. et al. Designed cell consortia as fragrance-programmable analog-to-digital converters. Nat. Chem. Biol.13, 309–316 (2017). - PubMed
MeSH terms
Substances
Grants and funding
- ElectroGene 785800/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- NCCR Molecular Systems Engineering/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
- Enlight/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- Fellowship/European Molecular Biology Organization (EMBO)
- 32071429/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources

