Lactate-to-albumin ratio as a potential prognostic predictor in patients with cirrhosis and sepsis: a retrospective cohort study
- PMID: 39953385
- PMCID: PMC11829571
- DOI: 10.1186/s12879-025-10601-6
Lactate-to-albumin ratio as a potential prognostic predictor in patients with cirrhosis and sepsis: a retrospective cohort study
Abstract
Background: Patients with liver cirrhosis face high infection risks due to immune dysfunction and hospital-related factors, increasing mortality rates when sepsis occurs. While various biomarkers predict outcomes in cirrhosis, few are accessible and reliable. This study addresses the gap by evaluating the prognostic potential of the lactate-to-albumin ratio (LAR).
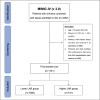
Methods: We retrospectively analyzed data from patients with cirrhosis and sepsis who were admitted to the intensive care unit at Beth Israel Deaconess Medical Center between 2008 and 2022. LAR was calculated from the ratio obtained from the first measurement taken within 24 h of admission. The optimal LAR threshold was determined using R statistical software. Kaplan-Meier analysis was used to compare mortality risks between two patient groups, while multivariate Cox proportional hazards regression models were used to assess the association between LAR and mortality risk in patients with cirrhosis and concomitant sepsis. A restricted cubic spline (RCS) was used to explore potential dose-response relationships between LAR and mortality. Receiver operating characteristic (ROC) analyses were used to assess the predictive ability, sensitivity, and specificity of LAR for all-cause mortality in patients with cirrhosis and combined sepsis, and the area under the curve (AUC) was calculated. Finally, subgroup analyses were performed to assess the relationship between LAR and prognosis across different populations.
Results: A total of 1731 patients were included in the study. The optimal LAR threshold was identified as 1.0 using R statistical software. Kaplan-Meier analysis indicated that patients with higher LAR levels had a higher risk of 14-day, 28-day, and 90-day all-cause mortality (all log-rank P < 0.001). Multivariate Cox proportional hazards models indicated independent associations between higher LAR levels and all-cause mortality at 14-day, 28-day, and 90-day before and after adjusting for confounders. RCS analysis revealed a nonlinear association between LAR and short- and long-term all-cause mortality in patients with cirrhosis and sepsis. ROC curve analysis showed that although the predictive value of LAR for the prognosis of patients with cirrhosis combined with sepsis was slightly inferior to that of the Model for End-Stage Liver Disease score, it was significantly better than that of lactate, albumin, and the Sequential Organ Failure Assessment. Subgroup analyses showed no significant interactions between LAR and any specific subgroup.
Conclusion: LAR has good predictive value for the prognosis of patients with cirrhosis and sepsis.
Keywords: Cirrhosis; Lactate to albumin ratio; Model for end-stage liver disease score; Prognosis; Sepsis; Sequential organ failure assessment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was conducted in accordance with the Declaration of Helsinki. The ethical review board of Beth Israel Deaconess Medical Center waived the requirement for patient informed consent as the study used de-identified data from the MIMIC-IV (v 3.0). The ethical review board of Beth Israel Deaconess Medical Center waived the requirement for formal ethics approval as the study used de-identified data, and no patient informed consent was required. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests. Clinical trial number: Not applicable.
Figures




Similar articles
-
Association between lactate-to-albumin ratio and mortality in hepatic failure: a retrospective cohort study.BMC Infect Dis. 2025 Mar 28;25(1):433. doi: 10.1186/s12879-025-10783-z. BMC Infect Dis. 2025. PMID: 40155840 Free PMC article.
-
Relationship between coagulopathy score and survival in critically ill patients with liver cirrhosis and sepsis: a retrospective study.BMC Infect Dis. 2025 Mar 26;25(1):418. doi: 10.1186/s12879-025-10848-z. BMC Infect Dis. 2025. PMID: 40140996 Free PMC article.
-
[Early lactate/albumin ratio combined with quick sequential organ failure assessment for predicting the prognosis of sepsis caused by community-acquired pneumonia in the emergency department].Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2025 Feb;37(2):118-122. doi: 10.3760/cma.j.cn121430-20240709-00577. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2025. PMID: 40017357 Chinese.
-
Lactate-enhanced-qSOFA (LqSOFA) score as a predictor of in-hospital mortality in patients with sepsis: systematic review and meta-analysis.Eur J Trauma Emerg Surg. 2025 Jan 24;51(1):33. doi: 10.1007/s00068-024-02757-8. Eur J Trauma Emerg Surg. 2025. PMID: 39853387
-
Lactate-to-Albumin Ratio (LAR) as a Predictor of All-Cause Mortality in Patients With Myocardial Infarction: A Systematic Review and Meta-Analysis.Cureus. 2025 Apr 13;17(4):e82166. doi: 10.7759/cureus.82166. eCollection 2025 Apr. Cureus. 2025. PMID: 40364881 Free PMC article. Review.
Cited by
-
Association between lactate-to-albumin ratio and mortality in hepatic failure: a retrospective cohort study.BMC Infect Dis. 2025 Mar 28;25(1):433. doi: 10.1186/s12879-025-10783-z. BMC Infect Dis. 2025. PMID: 40155840 Free PMC article.
-
Prognostic value of the lactate-to-albumin ratio in critically ill chronic heart failure patients with sepsis: insights from a retrospective cohort study.Front Med (Lausanne). 2025 Jul 15;12:1593524. doi: 10.3389/fmed.2025.1593524. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40735448 Free PMC article.
References
-
- GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol. 2020;5(3):245–66. 10.1016/S2468-1253(19)30349-8 - PMC - PubMed
-
- Durand F, Kellum JA, Nadim MK. Fluid resuscitation in patients with cirrhosis and sepsis: a multidisciplinary perspective. J Hepatol. 2023;79(1):240–6. 10.1016/j.jhep.2023.02.024 - PubMed
-
- Kosuta I, Premkumar M, Reddy KR. Review article: evaluation and care of the critically ill patient with cirrhosis. Aliment Pharmacol Ther. 2024;59(12):1489–509. 10.1111/apt.18016 - PubMed
MeSH terms
Substances
Grants and funding
- Grant No.2023HYX032/NHC Key Laboratory of Nuclear Technology Medical Transformation (Mianyang Central Hospital)
- Grant No.82400758/National Natural Science Foundation of China (NSFC)
- Grant no. 24QNMP028/Health Commission of Sichuan Province Medical Science and Technology Program
- Grant no. Q23046/Medical Research Youth Innovation Project of Sichuan Province, China
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

