Micheliolide attenuates sepsis-induced acute lung injury by suppressing mitochondrial oxidative stress and PFKFB3-driven glycolysis
- PMID: 39953547
- PMCID: PMC11829335
- DOI: 10.1186/s12967-024-05906-0
Micheliolide attenuates sepsis-induced acute lung injury by suppressing mitochondrial oxidative stress and PFKFB3-driven glycolysis
Abstract
Background: Sepsis is a potentially fatal condition with a significant risk of death. Acute lung injury (ALI) is a life-threatening complication of sepsis, and the inflammatory response plays a critical role in sepsis-induced ALI. The protective effects of micheliolide (MCL) against renal fibrosis and leukemia have been demonstrated, but the precise underlying mechanisms remain unclear.
Methods: In vitro, lipopolysaccharides (LPS) and interferon-gamma (IFN-γ) were used to stimulate RAW264.7 cells and bone marrow-derived macrophages (BMDMs) to investigate the protective effect of MCL on sepsis-induced ALI. Cecal ligation and puncture (CLP) models were constructed in mice to induce ALI in vivo. The expression of inflammatory factors, macrophage polarization markers, and the glycolysis-related enzyme 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3) were measured in vivo. Mitochondrial function, oxidative stress, and mitochondrial-related proteins were evaluated in vitro.
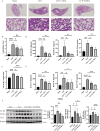
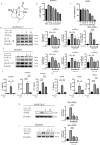
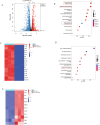
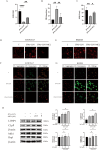
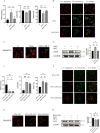
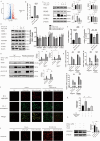
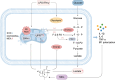
Results: MCL inhibited CLP-induced ALI, as evidenced by improvements in proinflammatory factor levels, lung wet/dry ratios, and histopathological findings. In vitro, MCL treatment significantly suppressed LPS + IFN-γ-induced M1-type polarization of RAW264.7 cells and BMDMs, as well as the production of inflammatory factors and oxidative stress. Mechanistic experiments revealed that MCL suppresses PFKFB3-driven glycolysis to reduce inflammation and activates the mitochondrial unfolded protein response (UPRmt) to alleviate mitochondrial stress. However, the therapeutic effect of MCL was diminished when PFKFB3 was overexpressed in cells.
Conclusion: This study is the first to demonstrate that MCL attenuates sepsis-induced ALI by reducing M1-type macrophage polarization. Its therapeutic effect is closely related to the suppression of oxidative stress and PFKFB3-driven glycolysis.
Keywords: Acute lung injury; Glycolysis; Macrophage polarization; Oxidative stress; PFKFB3.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All mice experiments were approved by the Southern Medical University Committee on Ethics of Animal Experiments (application NO: IACUC-LAC-20230705–001). Competing interests: The authors declare that they have no competing interests.
Figures

References
-
- Cheng B, Xie G, Yao S, Wu X, Guo Q, Gu M, Fang Q, Xu Q, Wang D, Jin Y, et al. Epidemiology of severe sepsis in critically ill surgical patients in ten university hospitals in China. Crit Care Med. 2007;35:2538–46. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

