Soluble TIM-3, likely produced by myeloid cells, predicts resistance to immune checkpoint inhibitors in metastatic clear cell renal cell carcinoma
- PMID: 39953623
- PMCID: PMC11827183
- DOI: 10.1186/s13046-025-03293-y
Soluble TIM-3, likely produced by myeloid cells, predicts resistance to immune checkpoint inhibitors in metastatic clear cell renal cell carcinoma
Abstract
Background: Immunotherapies targeting PD-1 and CTLA-4 are key components of the treatment of metastatic clear cell renal cell carcinoma (mccRCC). However, they have distinct safety profiles and resistance to treatment can occur. We assess soluble TIM-3 (sTIM-3) in the plasma of mccRCC patients as a potential theranostic biomarker, as well as its source and biological significance.
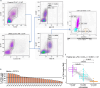
Methods: We analyzed the association between sTIM-3 and overall survival (OS), tumor response, and common clinical and biological factors in two mccRCC cohorts treated with anti-PD-1 (nivolumab, n = 27), anti-PD-1 or anti-PD-1 + anti-CTLA-4 (nivolumab + ipilimumab - N + I, n = 124). The origin and role of sTIM-3 are studied on tumor and blood samples, using multiplex immunohistochemistry and flow cytometry, as well as analyses of publicly available single-cell transcriptomic (scRNAseq) and mass cytometry data.
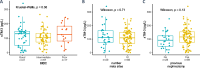
Results: sTIM-3 is significantly elevated in the plasma of treatment-naive mccRCC. It shows distinct associations with survival on anti-PD-1 vs anti-PD-1 + anti-CTLA-4: under nivolumab monotherapy, sTIM-3-high patients have a significantly reduced survival compared to sTIM-3-low patients, while they have similar survival probabilities under N + I. sTIM-3 is independent from other clinical and biological factors. Myeloid immune cells appear as the prominent source of sTIM-3, which may indicate their dysfunctional role in the antitumor immune response.
Conclusions: sTIM-3 appears to be a promising biomarker for optimizing treatment strategies in ccRCC as well as a potential therapeutic target, linked with to the immune myeloid compartment. Future investigations are warranted in patients treated with anti-PD-1 + antiangiogenic therapies.
Keywords: Biomarkers; Clear cell renal cell carcinoma; Immune checkpoints; Immunotherapy; Ipilimumab; Myeloid cells; Nivolumab; Soluble TIM-3.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The Colcheckpoint and BIONIKK cohorts have been approved by the French Health authorities and ethics committee [CPP Ile-de-France 8 (ref.16.10.69) and CPP Ouest I SI CNRIPH n18.11.21.67518 respectively]. All the participants provided written informed consent. Animal experiments were approved by the Ethics Committee of the University Paris Cité (CEEA34). Consent for publication: Not applicable. Competing interests: V.V. has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events and support for attending meetings and/or travel from MSD. Y.V. has received consulting fees from BMS, Ipsen, Eisai, MSD, Pfizer; research grants from BMS, Ipsen. S.O. has received consulting fees, payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events and support for attending meetings and/or travel from Pfizer, Novartis, Ipsen, Eisai, BMS, Merck; has participated in data safety monitoring board or advisory board from Roche, Ipsen, Eisai. The remaining authors declare that they have no competing interests.
Figures




References
-
- FDA C for DE and. Approved Drugs - Nivolumab (Opdivo Injection) - advanced renal cell carcinoma. Center for Drug Evaluation and Research. 2015. Available from: http://wayback.archive-it.org/7993/20170111231614/http://www.fda.gov/Dru.... Cited 18 Sep 2023.
-
- Powles T, Albiges L, Bex A, Comperat E, Grünwald V, Kanesvaran R, Kitamura H, McKay R, Porta C, Procopio G, Schmidinger M, Suarez C, Teoh J, de Velasco G, Young M, Gillessen S; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Renal cell carcinoma: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2024;35(8):692–706. 10.1016/j.annonc.2024.05.537. Epub 2024 May 22 - PubMed
-
- Motzer RJ, Rini BI, McDermott DF, Arén Frontera O, Hammers HJ, Carducci MA, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. 2019;20(10):1370–85. - PMC - PubMed
-
- Tang R, Rangachari M, Kuchroo VK. Tim-3: A co-receptor with diverse roles in T cell exhaustion and tolerance. Semin Immunol. 2019;42:101302. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials

