Ruthenium(II) Polypyridyl Complexes Containing COUBPY Ligands as Potent Photosensitizers for the Efficient Phototherapy of Hypoxic Tumors
- PMID: 39953993
- PMCID: PMC12164272
- DOI: 10.1021/jacs.4c15036
Ruthenium(II) Polypyridyl Complexes Containing COUBPY Ligands as Potent Photosensitizers for the Efficient Phototherapy of Hypoxic Tumors
Abstract
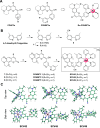
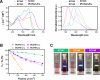
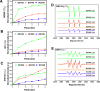
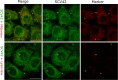
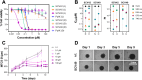
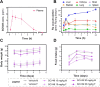
Hypoxia, a hallmark of many solid tumors, is linked to increased cancer aggressiveness, metastasis, and resistance to conventional therapies, leading to poor patient outcomes. This challenges the efficiency of photodynamic therapy (PDT), which relies on the generation of cytotoxic reactive oxygen species (ROS) through the irradiation of a photosensitizer (PS), a process partially dependent on oxygen levels. In this work, we introduce a novel family of potent PSs based on ruthenium(II) polypyridyl complexes with 2,2'-bipyridyl ligands derived from COUPY coumarins, termed COUBPYs. Ru-COUBPY complexes exhibit outstanding in vitro cytotoxicity against CT-26 cancer cells when irradiated with light within the phototherapeutic window, achieving nanomolar potency in both normoxic and hypoxic conditions while remaining nontoxic in the dark, leading to impressive phototoxic indices (>30,000). Their ability to generate both Type I and Type II ROS underpins their exceptional PDT efficiency. The lead compound of this study, SCV49, shows a favorable in vivo pharmacokinetic profile, excellent toxicological tolerability, and potent tumor growth inhibition in mice bearing subcutaneous CT-26 tumors at doses as low as 3 mg/kg upon irradiation with deep-red light (660 nm). These results allow us to propose SCV49 as a strong candidate for further preclinical development, particularly for treating large hypoxic solid tumors.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

