Real-world evidence of Carfilzomib, Lenalidomide and Dexamethasone (KRD) Scheme in patients with relapsed / refractory multiple myeloma
- PMID: 39954075
- PMCID: PMC11971156
- DOI: 10.1007/s00277-025-06240-1
Real-world evidence of Carfilzomib, Lenalidomide and Dexamethasone (KRD) Scheme in patients with relapsed / refractory multiple myeloma
Abstract
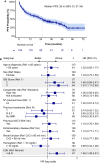
Carfilzomib, lenalidomide and dexamethasone (KRd) is still a widely used treatment option for patients with relapsed / refractory multiple myeloma (RRMM). However, there is limited real-world evidence. Here we evaluated the efficacy and safety of KRd in patients with RRMM treated following the standard clinical practice of the hospitals in the Catalan region. This was a retrospective, observational study. The objective response rate (ORR) according to IMWG criteria was the primary endpoint. Secondary endpoints included progression-free survival (PFS) and overall survival (OS). The study planned to include at least 100-150 patients. In total 194 patients were included. Median age was 64 years (range: 40-88) and 56% were male. All patients had received bortezomib. Additionally, 89 patients (46%) had received thalidomide and 29 (15%) lenalidomide. The ORR was 73% (95% CI: 66-79), with 72 patients (37%) having ≥ CR. The ORR was influenced by ISS score, number of previous treatments and exposure to lenalidomide. The median PFS was 26 months and 2-years OS rate 70%. The ISS stage and LDH levels were independent prognostic factors of survival. In conclusion, the KRd scheme led to a good effectiveness comparable safety profile to the phase III clinical trial in patients with RRMM.
Keywords: Carfilzomib, lenalidomide and dexamethasone; Real-world; Relapsed/refractory multiple myeloma.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: CFL: Advisory boards: Janssen, BMS, Amgen, Pfizer, Sanofi, Beigene, GSK, Roche, Menarini; Honoraria: Amgen, Janssen, BMS, GSK, Sanofi, Pfizer, Beigene; Grants: BMS, Janssen, Amgen, GSK.EC: Advisory boards and honoraria: Janssen, Sanofi, GSK, Menarini, Amgen.CM: Advisory boards: Sanofi.YG: Janssen and Sanofi.The remaining co-authors have no competing interests.
Figures



References
-
- Dimopoulos MA, Moreau P, Terpos E et al (2021) Multiple myeloma: EHA-ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol 32:309–322. 10.1016/j.annonc.2020.11.014 - PubMed
-
- Cavo M, Gay FM, Patriarca F et al (2017) Double autologous stem cell transplantation significantly prolongs progression-free survival and overall survival in comparison with single autotransplantation in newly diagnosed multiple myeloma: an analysis of phase 3 EMN02/HO95 study. Blood 130:401. 10.1182/blood.V130.Suppl_1.401.401
-
- Cavo M, Gay F, Beksac M et al (2020) Autologous haematopoietic stem-cell transplantation versus bortezomib-melphalan-prednisone, with or without bortezomib-lenalidomide-dexamethasone consolidation therapy, and lenalidomide maintenance for newly diagnosed multiple myeloma (EMN02/HO95): a multicentre, randomised, open-label, phase 3 study. Lancet Haematol 7:e456–e468. 10.1016/S2352-3026(20)30099-5 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

