Multilayer Adjuvanted Influenza Protein Nanoparticles Improve Intranasal Delivery and Antigen-Specific Immunity
- PMID: 39954231
- PMCID: PMC11867023
- DOI: 10.1021/acsnano.4c14735
Multilayer Adjuvanted Influenza Protein Nanoparticles Improve Intranasal Delivery and Antigen-Specific Immunity
Abstract
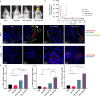
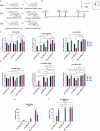
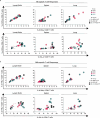
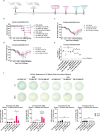
Intranasal vaccination is a desired route for protection against influenza viruses by mucosal and systemic immunity. However, the nasal mucosa impedes the intranasal delivery of vaccines. Here, we formulated layer-by-layer (LBL) influenza vaccine nanoparticles for effective intranasal delivery by coating them with alternating mucoadhesive cationic chitosan and muco-inert anionic CpG adjuvants. The nanoparticle cores were formed by desolvating influenza M2e antigen and coating it with hemagglutinin (HA) antigen via biotin-streptavidin conjugation. LBL modification promoted nasal delivery and interaction with the resident immune cells. Intranasal administration with LBL nanoparticles significantly improved cellular and humoral immune responses against HA and M2e including high IgA titers, a hallmark of potent mucosal immunity and persistence of immune responses. Distinct trends for antigen-specific immune responses were observed for different routes of vaccination. The enhanced immune responses conferred mice protection against the influenza challenge and prominently reduced viral titers, demonstrating the effectiveness of intranasal LBL vaccine nanoparticles.
Keywords: biodistribution; influenza vaccine; intranasal; layer-by-layer; mucosal immunology; nanoparticle; subunit vaccine.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Harper S. A.; Fukuda K.; Uyeki T. M.; Cox N. J.; Bridges C. B. Prevention and control of influenza: Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb. Mortal. Wkly. Rep. 2005, 54 (Rr-8), 1–40. - PubMed
-
- Oh J. E.; Song E.; Moriyama M.; Wong P.; Zhang S.; Jiang R.; Strohmeier S.; Kleinstein S. H.; Krammer F.; Iwasaki A. Intranasal priming induces local lung-resident B cell populations that secrete protective mucosal antiviral IgA. Science Immunology 2021, 6 (66), eabj512910.1126/sciimmunol.abj5129. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

