Lipid droplet efferocytosis attenuates proinflammatory signaling in macrophages via TREM2- and MS4A7-dependent mechanisms
- PMID: 39954254
- PMCID: PMC11973828
- DOI: 10.1016/j.celrep.2025.115310
Lipid droplet efferocytosis attenuates proinflammatory signaling in macrophages via TREM2- and MS4A7-dependent mechanisms
Abstract
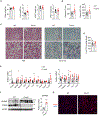
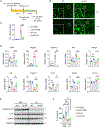
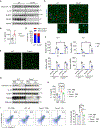
Metabolic dysfunction-associated steatohepatitis (MASH) is characterized by injury to steatotic hepatocytes that triggers the release of endogenous danger-associated molecular patterns. Recent work demonstrated that exposed lipid droplets (LDs) serve as a pathogenic signal that promotes monocyte infiltration and its maturation into triggering receptor expressed in myeloid cells 2 (TREM2+) macrophages in MASH liver. Here we explore the role of LD exposure in modulating inflammatory signaling in macrophages. We found that LD efferocytosis triggers a global transcriptional response and dampens pro-inflammatory signaling in macrophages. LD treatment attenuated NLRP3 inflammasome activation via mechanisms independent of lysosomal LD hydrolysis. While TREM2 was dispensable for LD efferocytosis by macrophages, it was required for the attenuation of proinflammatory signaling upon LD exposure. Additionally, MS4A7 downregulation contributes to LD efferocytosis-mediated dampening of inflammatory response. These results underscore the dual role of LD exposure in MASH liver by promoting monocyte infiltration and TREM2+ macrophage induction, while restraining proinflammatory response in macrophages.
Keywords: CP: Immunology; CP: Metabolism; MS4A7; NLRP3 inflammasome; TREM2; efferocytosis; inflammatory response; lipid droplets; macrophages.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

