Novel FAP-Targeted Heptamethine Cyanines for NIRF Imaging Applications
- PMID: 39954291
- PMCID: PMC11881144
- DOI: 10.1021/acs.molpharmaceut.4c01232
Novel FAP-Targeted Heptamethine Cyanines for NIRF Imaging Applications
Abstract
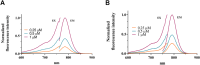
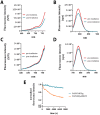
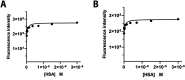
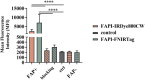
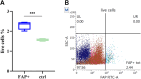
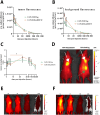
Fibroblast activation protein (FAP) is a pan-cancer target that is useful for imaging, ideally all epithelial cancers. This work aimed to develop, characterize, and validate two novel FAP-targeted probes for optical imaging, both in vitro and in vivo. IRDye800CW and FNIRTag heptamethine cyanines were conjugated to the NH precursor of the well-known FAP inhibitor FAPI-46, which is widely employed in nuclear medicine. In addition to synthesis, the dyes were characterized in terms of physicochemical properties, biodistribution, and imaging performances in a breast cancer tumor model. FAPI-FNIRTag showed a stronger fluorescence and higher photostability compared to FAPI-IRDye800CW. Notably, both compounds exhibited strong tumor accumulation in TUBO breast cancer-bearing mice 24 h postadministration, suggesting potential for further investigation as fluorescence-guided surgery (FGS) agents.
Keywords: FAP; breast cancer; heptamethine cyanines; near-infrared fluorescence imaging; optical imaging.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Kratochwil C.; Flechsig P.; Lindner T.; Abderrahim L.; Altmann A.; Mier W.; Adeberg S.; Rathke H.; Röhrich M.; Winter H.; Plinkert P. K.; Marme F.; Lang M.; Kauczor H. U.; Jäger D.; Debus J.; Haberkorn U.; Giesel F. L. 68Ga-FAPI PET/CT: Tracer uptake in 28 different kinds of cancer. J. Nucl. Med. 2019, 60, 801–805. 10.2967/jnumed.119.227967. - DOI - PMC - PubMed
-
- Sandberg T. P.; Stuart M. P. M. E.; Oosting J.; Tollenaar R. A. E. M.; Sier C. F. M.; Mesker W. E. Increased expression of cancer-associated fibroblast markers at the invasive front and its association with tumor-stroma ratio in colorectal cancer. BMC Cancer. 2019, 19, 28410.1186/s12885-019-5462-2. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

