Self supervised artificial intelligence predicts poor outcome from primary cutaneous squamous cell carcinoma at diagnosis
- PMID: 39955424
- PMCID: PMC11830021
- DOI: 10.1038/s41746-025-01496-3
Self supervised artificial intelligence predicts poor outcome from primary cutaneous squamous cell carcinoma at diagnosis
Abstract
Primary cutaneous squamous cell carcinoma (cSCC) is responsible for ~10,000 deaths annually in the United States. Stratification of risk of poor outcome at initial biopsy would significantly impact clinical decision-making during the initial post operative period where intervention has been shown to be most effective. Using whole-slide images (WSI) from 163 patients from 3 institutions, we developed a self supervised deep-learning model to predict poor outcomes in cSCC patients from histopathological features at initial diagnosis, and validated it using WSI from 563 patients, collected from two other academic institutions. For disease-free survival prediction, the model attained a concordance index of 0.73 in the development cohort and 0.84 in the Mayo cohort. The model's interpretability revealed that features like poor differentiation and deep invasion were strongly associated with poor prognosis. Furthermore, the model is effective in stratifying risk among BWH T2a and AJCC T2, known for outcome heterogeneity.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare the following competing interests: A.T. is a co-founder of Imagenomix; N.C. is a scientific advisor for Imagenomix. The other authors declare that they have no competing interests.
Figures


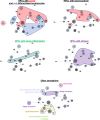
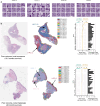


Update of
-
Self-supervised artificial intelligence predicts recurrence, metastasis and disease specific death from primary cutaneous squamous cell carcinoma at diagnosis.Res Sq [Preprint]. 2023 Dec 13:rs.3.rs-3607399. doi: 10.21203/rs.3.rs-3607399/v1. Res Sq. 2023. Update in: NPJ Digit Med. 2025 Feb 15;8(1):105. doi: 10.1038/s41746-025-01496-3. PMID: 38168253 Free PMC article. Updated. Preprint.
References
-
- Lomas, A., Leonardi-Bee, J. & Bath-Hextall, F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br. J. Dermatol.166, 1069–1080 (2012). - PubMed
-
- Waldman, A. & Schmults, C. Cutaneous Squamous Cell Carcinoma. Hematol. Oncol. Clin. North Am.33, 1–12 (2019). - PubMed
-
- Leiter, U. et al. Incidence, Mortality, and Trends of Nonmelanoma Skin Cancer in Germany. J. Invest. Dermatol.137, 1860–1867 (2017). - PubMed
-
- Van Lee, C. B. et al. Recurrence rates of cutaneous squamous cell carcinoma of the head and neck after Mohs micrographic surgery vs. standard excision: a retrospective cohort study. Br. J. Dermatol.181, 338–343 (2019). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

