Human breast milk-derived exosomes attenuate lipopolysaccharide-induced activation in microglia
- PMID: 39955566
- PMCID: PMC11830176
- DOI: 10.1186/s12974-025-03345-2
Human breast milk-derived exosomes attenuate lipopolysaccharide-induced activation in microglia
Abstract
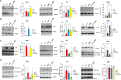
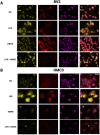
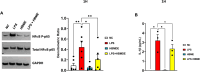
Microglia mediate the immune response in the central nervous system to many insults, including lipopolysaccharide (LPS), a bacterial endotoxin that initiates neuroinflammation in the neonatal population, especially preterm infants. The synthesis of the proinflammatory proteins CD40 and NLRP3 depends on the canonical NF-κB cascade as the genes encoding CD40 and NLRP3 are transcribed by the phosphorylated NF-κB p50/p65 heterodimer in LPS-induced microglia. Exosomes, which are nanosized vesicles (40-150 nm) involved in intercellular communication, are implicated in many pathophysiological processes. Human breast milk, which is rich in exosomes, plays a vital role in neonatal immune system maturation and adaptation. Activated microglia may cause brain-associated injuries or disorders; therefore, we hypothesize that human breast milk-derived exosomes (HBME) attenuate LPS-induced activation of CD40 and NLRP3 by decreasing p38 MAPK and NF-κB p50/p65 activation/phosphorylation downstream of TLR4 in murine microglia (BV2). Human microglia (HMC3) showed a significant decrease in p65 phosphorylation. We isolated purified HBME and characterized them using nanoparticle tracking analysis, transmission electron microscopy, fluorescence-activated cell sorting, and western blots. Analysis of microglia exposed to LPS and HBME indicated that HBME modulated the expression of signaling molecules in the canonical NF-κB pathway, including MyD88, IκBα, p38 MAPK, NF-κB p65, and their products CD40, NLRP3, and cytokines IL-1β and IL-10. Thus, HBMEs have great potential for attenuating the microglial response to LPS.
Keywords: BV2; Breast milk; CD40; Exosomes; HMC3; IL-10; IL-1β; Lipopolysaccharide; Microglia; NFκB; NLRP3; Neonatal neuroinflammation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Ethics approval and consent to participate were not needed. BV2 cells were a generous gift from Dr. Harald Neumann at the University of Bonn LIFE and Brain Center in Bonn, Germany. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures




References
-
- Zhou Q, Guo D, Li X, Wang Y, Ye X, Xue S, et al. Anti-inflammatory effects of vinpocetine in LPS-stimulated microglia via activation of AMPK. An Acad Bras Ciênc. 2020;92(4): e20200241. - PubMed
-
- Shemer A, Scheyltjens I, Frumer GR, Kim J-S, Grozovski J, Ayanaw S, et al. Interleukin-10 prevents pathological microglia hyperactivation following peripheral endotoxin challenge. Immunity. 2020;53(5):1033-49.e7. - PubMed
-
- Dean JM, van de Looij Y, Sizonenko SV, Lodygensky GA, Lazeyras F, Bolouri H, et al. Delayed cortical impairment following lipopolysaccharide exposure in preterm fetal sheep. Ann Neurol. 2011;70(5):846–56. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

