Innate immune sensors and regulators at the blood brain barrier: focus on toll-like receptors and inflammasomes as mediators of neuro-immune crosstalk and inflammation
- PMID: 39955600
- PMCID: PMC11829548
- DOI: 10.1186/s12974-025-03360-3
Innate immune sensors and regulators at the blood brain barrier: focus on toll-like receptors and inflammasomes as mediators of neuro-immune crosstalk and inflammation
Abstract
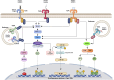
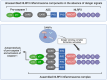
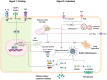
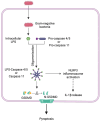
Cerebral endothelial cells (CEC) that form the brain capillaries are the principal constituents of the blood brain barrier (BBB), the main active interface between the blood and the brain which plays a protective role by restricting the infiltration of pathogens, harmful substances and immune cells into the brain while allowing the entry of essential nutrients. Aberrant CEC function often leads to increased permeability of the BBB altering the bidirectional communication between the brain and the bloodstream and facilitating the extravasation of immune cells into the brain. In addition to their role as essential gatekeepers of the BBB, CEC exhibit immune cell properties as they can receive and transmit signals between the blood and the brain partly via release of inflammatory effectors in pathological conditions. Cerebral endothelial cells express innate immune receptors, including toll like receptors (TLRs) and inflammasomes which are the first sensors of exogenous or endogenous dangers and initiators of immune and inflammatory responses which drive neural dysfunction and degeneration. Accumulating evidence indicates that activation of TLRs and inflammasomes in CEC compromises BBB integrity, promotes aberrant neuroimmune interactions and modulates both systemic and neuroinflammation, common pathological features of neurodegenerative and psychiatric diseases and central nervous system (CNS) infections and injuries. The goal of the present review is to provide an overview of the pivotal roles played by TLRs and inflammasomes in CEC function and discuss the molecular and cellular mechanisms by which they contribute to BBB disruption and neuroinflammation especially in the context of traumatic and ischemic brain injuries and brain infections. We will especially focus on the most recent advances and literature reports in the field to highlight the knowledge gaps. We will discuss future research directions that can advance our understanding of the central contribution of innate immune receptors to CEC and BBB dysfunction and the potential of innate immune receptors at the BBB as promising therapeutic targets in a wide variety of pathological conditions of the brain.
Keywords: Blood brain barrier; Brain injury; COVID-19.; Endothelial cells; Infection; Inflammasome; Neurovascular unit; Pericytes; Stroke; Toll like receptors.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and Consent for Participation: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
-
- Lizano P, Pong S, Santarriaga S, Bannai D, Karmacharya R. Brain microvascular endothelial cells and blood-brain barrier dysfunction in psychotic disorders. Mol Psychiatry. 2023;28:3698–708. - PubMed
-
- Segarra M, Aburto MR, Acker-Palmer A. Blood-brain barrier dynamics to maintain brain homeostasis. Trends Neurosci. 2021;44:393–405. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

