A Novel Two-Part Mixture Model for the Incidence and Time Course of Cytokine Release Syndrome After Elranatamab Dosing in Multiple Myeloma Patients
- PMID: 39955765
- PMCID: PMC12087694
- DOI: 10.1002/cpt.3533
A Novel Two-Part Mixture Model for the Incidence and Time Course of Cytokine Release Syndrome After Elranatamab Dosing in Multiple Myeloma Patients
Abstract
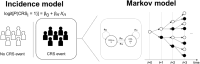
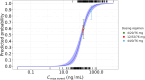
Cytokine release syndrome (CRS) is a common, acute adverse event associated with T-cell redirecting therapies such as bispecific antibodies (BsAbs). The nature of CRS events data makes it challenging to capture an unbiased exposure-response relationship with commonly used models. For example, simple logistic regression models cannot handle traditional time-varying exposure, and static exposure metrics chosen at early time points and with lower priming doses may underestimate the incidence of CRS. Therefore, more advanced modeling techniques are needed to adequately describe the time course of BsAb-induced CRS. Herein, we present a two-part mixture model that describes the population incidence and time course of CRS following various dose-priming regimens of elranatamab, a humanized BsAb that targets the B-cell maturation antigen on myeloma cells and CD3 on T cells, where the conditional time-evolution of CRS was described with a two-state (i.e., CRS-yes or no) Markov model. In the first part, increasing elranatamab exposure (maximum elranatamab concentration at first CRS event time (Cmax,event)) was associated with an increased CRS incidence probability. Similarly, in the second part, increased early elranatamab exposure (Cmax,D1) increased the predicted probability of CRS over time, whereas premedication including corticosteroids and IL-6 pathway inhibitors use demonstrated the opposite effect. This is the first reported application of a Markov model to describe the probability of CRS following BsAb therapy, and it successfully explained differences between different dose-priming regimens via clinically relevant covariates. This approach may be useful for the future clinical development of BsAbs.
© 2025 The Author(s). Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
D.I., J.H., M.E., D.W., E.V., K.P., and B.S. are full‐time employees of Pfizer, Inc., and own Pfizer stock. J.W. was a full‐time employee of Pfizer, Inc. at the time of conducting this analysis and may own Pfizer stock.
Figures



References
-
- Li, J. et al. CD3 bispecific antibody‐induced cytokine release is dispensable for cytotoxic T cell activity. Sci. Transl. Med. 11, eaax8861 (2019). - PubMed
-
- Lee, D.W. et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Transplant. 25, 625–638 (2019). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

