Adherence to Treatment in Allergic Rhinitis During the Pollen Season in Europe: A MASK-air Study
- PMID: 39956639
- PMCID: PMC11908838
- DOI: 10.1111/cea.70004
Adherence to Treatment in Allergic Rhinitis During the Pollen Season in Europe: A MASK-air Study
Erratum in
-
Correction to "Adherence to Treatment in Allergic Rhinitis During the Pollen Season in Europe: A MASK-air Study".Clin Exp Allergy. 2025 Jul;55(7):593. doi: 10.1111/cea.70041. Epub 2025 Mar 24. Clin Exp Allergy. 2025. PMID: 40129182 Free PMC article. No abstract available.
Abstract
Background: Adherence to rhinitis treatment has been insufficiently assessed. We aimed to use data from the MASK-air mHealth app to assess adherence to oral antihistamines (OAH), intra-nasal corticosteroids (INCS) or azelastine-fluticasone in patients with allergic rhinitis.
Methods: We included regular European MASK-air users with self-reported allergic rhinitis and reporting at least 1 day of OAH, INCS or azelastine-fluticasone. We assessed weeks during which patients answered the MASK-air questionnaire on all days. We restricted our analyses to data provided between January and June, to encompass the pollen seasons across the different assessed countries. We analysed symptoms using visual analogue scales (VASs) and the combined symptom-medication score (CSMS), performing stratified analyses by weekly adherence levels. Medication adherence was computed as the proportion of days in which patients reported rhinitis medication use. Sensitivity analyses were performed considering all weeks with at most 1 day of missing data and all months with at most 4 days of missing data.
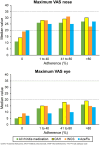
Results: We assessed 8212 complete weeks (1361 users). Adherence (use of medication > 80% days) to specific drug classes ranged from 31.7% weeks for azelastine-fluticasone to 38.5% weeks for OAH. Similar adherence to rhinitis medication was found in users with or without self-reported asthma, except for INCS (better adherence in asthma patients). VAS and CSMS levels increased from no adherence to full adherence, except for INCS. A higher proportion of days with uncontrolled symptoms was observed in weeks with higher adherence. In full adherence weeks, 41.2% days reported rhinitis co-medication. The sensitivity analyses displayed similar results.
Conclusions: A high adherence was found in patients reporting regular use of MASK-air. Different adherence patterns were found for INCS compared to OAH or azelastine-fluticasone that are likely to impact guidelines.
Keywords: allergic rhinitis; mobile health; treatment adherence.
© 2025 The Author(s). Clinical & Experimental Allergy published by John Wiley & Sons Ltd.
Conflict of interest statement
JB reports personal fees from Cipla, Menarini, Mylan, Novartis, Purina, Sanofi‐Aventis, Teva, Noucor, other from KYomed‐Innov, other from Mask‐air‐SAS, outside the submitted work. ICO reports other from Sanofi‐Aventis, outside the submitted work. PD reports personal fees and non‐financial support from Astra Zeneca, personal fees from Chiesi, personal fees and non‐financial support from Boehringer Ingelheim, personal fees from GlaxoSmithKline, personal fees from Menarini, personal fees and non‐financial support from Stallergenes, personal fees and non‐financial support from ALK Abello, outside the submitted work. MM reports other from ASTRA ZENECA, other from SANOFI AVENTIS, other from PFIZER, personal fees from TAKEDA, other from CHIESI, outside the submitted work. OP reports grants and personal fees from ALK‐Abelló, grants and personal fees from Allergopharma, grants and personal fees from Stallergenes Greer, grants and personal fees from HAL Allergy Holding B.V./HAL Allergie GmbH, grants from Bencard Allergie GmbH/Allergy Therapeutics, grants from Lofarma, grants and personal fees from ASIT Biotech Tools S.A., grants and personal fees from Laboratorios LETI/LETI Pharma, grants and personal fees from GlaxoSmithKline, personal fees from ROXALL Medizin, personal fees from Novartis, grants and personal fees from Sanofi‐Aventis and Sanofi‐Genzyme, personal fees from Med Update Europe GmbH, personal fees from streamedup! GmbH, grants from Pohl‐Boskamp, grants from Inmunotek S.L., personal fees from John Wiley and Sons, AS, personal fees from Paul‐Martini‐Stiftung (PMS), personal fees from Regeneron Pharmaceuticals Inc., personal fees from RG Aerztefortbildung, personal fees from Institut für Disease Management, personal fees from Springer GmbH, grants and personal fees from AstraZeneca, personal fees from IQVIA Commercial, personal fees from Ingress Health, personal fees from Wort&Bild Verlag, personal fees from Verlag ME, personal fees from Procter&Gamble, personal fees from ALTAMIRA, personal fees from Meinhardt Congress GmbH, personal fees from Deutsche Forschungsgemeinschaft, personal fees from Thieme, grants from Deutsche AllergieLiga e.V., personal fees from AeDA, personal fees from Alfried‐Krupp Krankenhaus, personal fees from Red Maple Trials Inc., personal fees from Königlich Dänisches Generalkonsulat, personal fees from Medizinische Hochschule Hannover, personal fees from ECM Expro&Conference Management, personal fees from Technical University Dresden, personal fees from Lilly, personal fees from Paul Ehrlich Institut, personal fees from Japanese Society of Allergy, personal fees from Forum für Medizinische Fortbildung, from Dustri‐Verlag, outside the submitted work; and member of EAACI Excom, member of ext. board of directors DGAKI; coordinator, main‐ or co‐author of different position papers and guidelines in rhinology, allergology and allergen‐immunotherapy. VK reports non‐financial support from NORAMEDA, non‐financial support from DIMUNA, non‐financial support from Berlin CHemie Menarini, outside the submitted work. TH reports personal fees from Orion Pharma, outside the submitted work. DLL reports personal fees from ALK, Astrazeneca national and global, Bayer, Chiesi, Grunenthal, Grin, GSK national and global, Viatris, Menarini, MSD, Novartis, Pfizer, Sanofi, Siegfried, UCB, Carnot, grants from Abbvie, Bayer, Lilly, Sanofi, Astrazeneca, Pfizer, Novartis, Circassia, UCB, GSK., outside the submitted work. NP reports grants from Capricare, personal fees from Nestle, personal fees from Numil, personal fees from Vianex, personal fees from REG, outside the submitted work. JCI reports personal fees from Laboratorios Casasco, personal fees from Bago Bolivia, personal fees from Abbott Ecuador, personal fees from Faes Farma, outside the submitted work. LTB reports personal fees from AstraZeneca, personal fees from LETI Laboratories, personal fees from Sanofi, outside the submitted work. LC reports personal fees from Novartis, personal fees from Astra Zeneca, personal fees from GSK, personal fees from ALK, personal fees from Thermofisher, personal fees from Sanofi, outside the submitted work. RL reports grants and personal fees from GSK, grants and personal fees from AZ, grants from Chiesi, outside the submitted work. HK reports personal fees from Sanofi, personal fees from Berlin‐Chemie, personal fees from Viatris/Mylan, outside the submitted work. BS reports personal fees from Polpharma, personal fees from Viatris, grants and personal fees from AstraZeneca, personal fees from TEVA, personal fees from patient ombudsman, personal fees from Polish Allergology Society, grants from GSK, personal fees from ADAMED, outside the submitted work. MZ reports personal fees from Takeda, outside the submitted work. IA reports personal fees from Abbott, personal fees from Bayer, personal fees from Bial, personal fees from Eurodrug, personal fees from Faes Farma, personal fees from Gebro, personal fees from Menarini, personal fees from MSD, personal fees from Roxall, personal fees from Sanofi, outside the submitted work. JS reports grants and personal fees from SANOFI, personal fees from GSK, personal fees from NOVARTIS, personal fees from ASTRA ZENECA, personal fees from MUNDIPHARMA, personal fees from FAES FARMA, outside the submitted work. STS reports grants and other from Sanofi, grants and other from GSK, other from Clario, other from Orion Pharma, other from ALK‐Abelló, other from Roche, other from AstraZeneca, outside the submitted work. TZ reports grants and personal fees from Novartis, grants and personal fees from Henkel, personal fees from Bayer, personal fees from FAES, personal fees from Astra Zeneca, personal fees from AbbVie, personal fees from ALK, personal fees from Almirall, personal fees from Astellas, personal fees from Bayer, personal fees from Bencard, personal fees from Berlin Chemie, personal fees from FAES, personal fees from Hal, personal fees from Leti, personal fees from Mesa, personal fees from Menarini, personal fees from Merck, personal fees from MSD, personal fees from Novartis, personal fees from Pfizer, personal fees from Sanofi, personal fees from Stallergenes, personal fees from Takeda, personal fees from Teva, personal fees from UCB, personal fees from Henkel, personal fees from Kryolan, personal fees from L'Oreal, outside the submitted work; and Organisational affiliations: Committee member: WHO‐Initiative ‘Allergic Rhinitis and Its Impact on Asthma’ (ARIA); Member of the Board: German Society for Allergy and Clinical Immunology (DGAKI); Head: European Centre for Allergy Research Foundation (ECARF); President: Global Allergy and Asthma European Network (GA2LEN); Member: Committee on Allergy Diagnosis and Molecular Allergology, World Allergy Organisation (WAO). AC reports personal fees from AstraZeneca, personal fees from Boehringer‐Ingelheim, personal fees from Chiesi, personal fees from GSK, personal fees from Sanofi, personal fees from Eurofarma, personal fees from Novartis, personal fees from Crossject, personal fees from Glennmark, personal fees from Abdi Ibrahim, personal fees from Mylan, personal fees from Farmoquimica, personal fees from Ache, outside the submitted work. PK reports personal fees from Adamed, personal fees and other from Berlin Chemie Menarini, personal fees from Boehringer Ingelheim, personal fees from AstraZeneca, personal fees from Celon Pharma, personal fees from FAES, personal fees from Novartis, personal fees from Polpharma, personal fees from GSK, personal fees from Sanofi, personal fees from Teva, personal fees from Zentiva, outside the submitted work. The other authors have no COI to disclose, outside the submitted work.
Figures
References
-
- Brozek J. L., Bousquet J., Agache I., et al., “Allergic Rhinitis and Its Impact on Asthma (ARIA) Guidelines‐2016 Revision,” Journal of Allergy and Clinical Immunology 140, no. 4 (2017): 950–958. - PubMed
-
- Dykewicz M. S., Wallace D. V., Amrol D. J., et al., “Rhinitis 2020: A Practice Parameter Update,” Journal of Allergy and Clinical Immunology 146, no. 4 (2020): 721–767. - PubMed
-
- Braido F., Baiardini I., Puggioni F., Garuti S., Pawankar R., and Walter Canonica G., “Rhinitis: Adherence to Treatment and New Technologies,” Current Opinion in Allergy and Clinical Immunology 17, no. 1 (2017): 23–27. - PubMed
-
- Passalacqua G., Baiardini I., Senna G., and Canonica G. W., “Adherence to Pharmacological Treatment and Specific Immunotherapy in Allergic Rhinitis,” Clinical and Experimental Allergy 43, no. 1 (2013): 22–28. - PubMed
-
- Sousa‐Pinto B., Sa‐Sousa A., Vieira R. J., et al., “Behavioural Patterns in Allergic Rhinitis Medication in Europe: A Study Using MASK‐Air((R)) Real‐World Data,” Allergy 77, no. 9 (2022): 2699–2711. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical