Baseline characteristics of contemporary trial participants with heart failure and reduced ejection fraction: The VICTOR trial
- PMID: 39956649
- PMCID: PMC12482839
- DOI: 10.1002/ejhf.3598
Baseline characteristics of contemporary trial participants with heart failure and reduced ejection fraction: The VICTOR trial
Abstract
Aims: To describe the baseline characteristics of the participants in the VICTOR (Vericiguat Global Study in Patients with Chronic Heart Failure; NCT05093933) trial and compare them to recent trials in patients with heart failure and reduced ejection fraction (HFrEF).
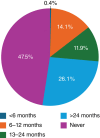
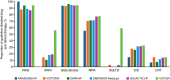
Methods and results: Baseline characteristics were evaluated in 6105 patients randomized to vericiguat or placebo. The mean age of the participants was 67 ± 11 years, 23.6% were women, 64.4% were White, and 10.7% were self-identified Black. Overall, 29.1% of participants were enrolled in Latin and South America, 27.8% and 18.5% in Eastern and Western Europe, 14.0% in Asia-Pacific and 10.6% in North America. The mean left ventricular ejection fraction was 30 ± 7% and 79% had New York Heart Association class II symptoms. Mean estimated glomerular filtration rate was 70.9 ± 24.0 ml/min/1.73 m2. Median N-terminal pro-B-type natriuretic peptide level was 1476 (970-2495) pg/ml and 47.5% of participants had no prior hospitalization for heart failure. Baseline therapy included 94.4% beta-blockers, 94.3% renin-angiotensin system modulation (including 56.0% on an angiotensin receptor-neprilysin inhibitor), 77.7% mineralocorticoid receptor antagonists, 59.1% sodium-glucose cotransporter 2 inhibitors, and 32.9% implantable cardioverter-defibrillators. Overall characteristics were relatively similar to other recent HFrEF trials but the VICTOR trial enrolled a higher proportion of participants receiving contemporary drug and device therapy.
Conclusion: The VICTOR trial enrolled the most optimally treated population in a phase III heart failure trial. These features will facilitate assessment of the benefit of vericiguat and elucidate the outcomes of patients on up-to-date medical therapy.
Keywords: Clinical characteristics; Heart failure; Treatment; Vericiguat.
© 2025 Merck Sharp & Dohme LLC. Bayer AG and The Author(s). European Journal of Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Figures
References
-
- Crespo‐Leiro MG, Anker SD, Maggioni AP, Coats AJ, Filippatos G, Ruschitzka F, et al.; Heart Failure Association (HFA) of the European Society of Cardiology (ESC) . European Society of Cardiology Heart Failure Long‐Term Registry (ESC‐HF‐LT): 1‐year follow‐up outcomes and differences across regions. Eur J Heart Fail 2016;18:613–625. 10.1002/ejhf.566 - DOI - PubMed
-
- Johansson I, Joseph P, Balasubramanian K, McMurray JJV, Lund LH, Ezekowitz JA, et al.; G‐CHF Investigators . Health‐related quality of life and mortality in heart failure: the Global Congestive Heart Failure study of 23 000 patients from 40 countries. Circulation 2021;143:2129–2142. 10.1161/CIRCULATIONAHA.120.050850 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

