Tissue factor-dependent colitogenic CD4+ T cell thrombogenicity is regulated by activated protein C signalling
- PMID: 39956825
- PMCID: PMC11830781
- DOI: 10.1038/s41467-025-57001-7
Tissue factor-dependent colitogenic CD4+ T cell thrombogenicity is regulated by activated protein C signalling
Abstract
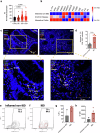
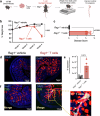
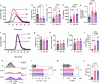
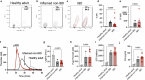
Patients with inflammatory bowel disease (IBD) have an increased risk of venous thromboembolism (VTE), but the underlying mechanistic basis remains poorly defined. Here, we find that colitogenic CD4+ T cells express tissue factor (TF) and promote rapid TF-dependent plasma thrombin generation. TF+CD3+CD4+ T cells are present in both the colons of mice with experimental colitis and blood and colonic tissue from patients with IBD. Expression of genes involved in regulating coagulation, including Protein C (PC; encoded by PROC) and its receptor (PROCR), are dysregulated in IBD patient gut biopsy tissues. Moreover, activated PC signalling reduces the procoagulant activity mediated by TF+CD4+ T cells. Our data thus identify TF-induced, colitogenic T cell-mediated thrombogenicity, and also demonstrate a new function for activated PC signalling in regulating T cell thrombo-inflammatory activity.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


References
-
- European Federation of Crohns & Ulcerative Colitis Associations (EFFCA). What is IBD?https://efcca.org (EFFCA, 2024).
-
- Kappelman, M. D. et al. Thromboembolic risk among Danish children and adults with inflammatory bowel diseases: a population-based nationwide study. Gut60, 937–943 (2011). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

