Microbiota-derived IPA alleviates intestinal mucosal inflammation through upregulating Th1/Th17 cell apoptosis in inflammatory bowel disease
- PMID: 39956891
- PMCID: PMC11834480
- DOI: 10.1080/19490976.2025.2467235
Microbiota-derived IPA alleviates intestinal mucosal inflammation through upregulating Th1/Th17 cell apoptosis in inflammatory bowel disease
Abstract
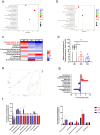
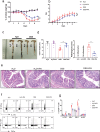
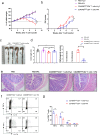
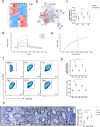
The gut microbiota-derived metabolite indole-3-propionic acid (IPA) plays an important role in maintaining intestinal mucosal homeostasis, while the molecular mechanisms underlying IPA regulation on mucosal CD4+ T cell functions in inflammatory bowel disease (IBD) remain elusive. Here we investigated the roles of IPA in modulating mucosal CD4+ T cells and its therapeutic potential in treatment of human IBD. Leveraging metabolomics and microbial community analyses, we observed that the levels of IPA-producing microbiota (e.g. Peptostreptococcus, Clostridium, and Fournierella) and IPA were decreased, while the IPA-consuming microbiota (e.g. Parabacteroides, Erysipelatoclostridium, and Lachnoclostridium) were increased in the feces of IBD patients than those in healthy donors. Dextran sulfate sodium (DSS)-induced acute colitis and CD45RBhighCD4+ T cell transfer-induced chronic colitis models were then established in mice and treated orally with IPA to study its role in intestinal mucosal inflammation in vivo. We found that oral administration of IPA attenuated mucosal inflammation in both acute and chronic colitis models in mice, as characterized by increased body weight, and reduced levels of pro-inflammatory cytokines (e.g. TNF-α, IFN-γ, and IL-17A) and histological scores in the colon. We further utilized RNA sequencing, molecular docking simulations, and surface plasmon resonance analyses and identified that IPA exerts its biological effects by interacting with heat shock protein 70 (HSP70), leading to inducing Th1/Th17 cell apoptosis. Consistently, ectopic expression of HSP70 in CD4+ T cells conferred resistance to IPA-induced Th1/Th17 cell apoptosis. Therefore, these findings identify a previously unrecognized pathway by which IPA modulates intestinal inflammation and provide a promising avenue for the treatment of IBD.
Keywords: HSP70; Indole-3-propionic acid; Th1 cells; Th17 cells; mucosal inflammation.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
