Quantifying antibody binding: techniques and therapeutic implications
- PMID: 39957177
- PMCID: PMC11834528
- DOI: 10.1080/19420862.2025.2459795
Quantifying antibody binding: techniques and therapeutic implications
Abstract
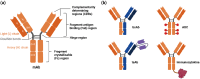
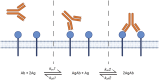
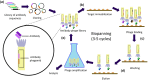
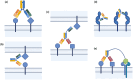
The binding kinetics of an antibody for its target antigen represent key determinants of its biological function and success as a novel biotherapeutic. Defining these interactions and kinetics is critical for understanding the pharmacological and pharmacodynamic profiles of antibodies in therapeutic applications, with line of sight to clinical translation. In this review, we discuss the latest developments in approaches to measure and modulate antibody-antigen interactions, including antibody engineering, novel antibody formats, current, and emerging technologies for measuring antibody-antigen binding interactions, and emerging perspectives within the field. We also explore how emerging computational methods are set to become powerful tools for modeling antibody-binding interactions under physiologically relevant conditions. Finally, we consider the therapeutic implications of modulating target engagement in terms of pharmacodynamics and pharmacokinetics.
Keywords: Antibody; affinity; avidity; pharmacokinetics; pharmacology; target engagement.
Conflict of interest statement
J.L, L.K., J.C., and J.W.T.Y. are employees and stock-holders of GSK.
Figures
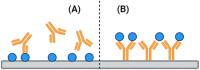
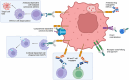
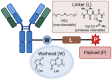
References
-
- Morgan P, Van Der Graaf PH, Arrowsmith J, Feltner DE, Drummond KS, Wegner CD, Street SDA. Can the flow of medicines be improved? Fundamental pharmacokinetic and pharmacological principles toward improving Phase II survival. Drug Discov Today. 2012;17(9–10):419–424. doi:10.1016/j.drudis.2011.12.020. - DOI - PubMed
-
- Tembhare PR, Marti G, Wiestner A, Degheidy H, Farooqui M, Kreitman RJ, Jasper GA, Yuan CM, Liewehr D, Venzon D, et al. Quantification of expression of antigens targeted by antibody-based therapy in chronic lymphocytic leukemia. Am J Clin Pathol. 2013;140(6):813–818. doi:10.1309/AJCPYFQ4XMGJD6TI. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
