Structurally targeted mutagenesis identifies key residues supporting α-synuclein misfolding in multiple system atrophy
- PMID: 39957201
- PMCID: PMC11924605
- DOI: 10.3233/JPD-240296
Structurally targeted mutagenesis identifies key residues supporting α-synuclein misfolding in multiple system atrophy
Abstract
Background: Multiple system atrophy (MSA) and Parkinson's disease (PD) are caused by misfolded α-synuclein spreading throughout the central nervous system. While familial PD is linked to several α-synuclein mutations, no mutations are associated with MSA. We previously showed that the familial PD mutation E46K inhibits replication of MSA prions both in vitro and in vivo, providing key evidence to support the hypothesis that α-synuclein adopts unique strains in patients.
Objective: Here we sought to further interrogate α-synuclein misfolding to identify the structural determinants that contribute to MSA strain biology.
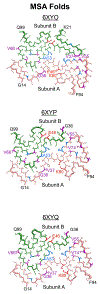
Methods: We engineered a panel of cell lines harbouring both PD-linked and novel mutations designed to identify key residues that facilitate α-synuclein misfolding in MSA. We also used Maestro in silico analyses to predict the effect of each mutation on α-synuclein misfolding into one of the reported MSA cryo-electron microscopy conformations.
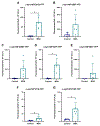
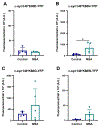
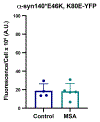
Results: In many cases, our modelling accurately identified mutations that facilitated or inhibited MSA replication. However, Maestro was occasionally unable to predict the effect of a mutation, demonstrating the challenge of using computational tools to investigate intrinsically disordered proteins. Finally, we used our cellular models to determine the mechanism underlying the E46K-driven inhibition of MSA replication, finding that the E46/K80 salt bridge is necessary to support α-synuclein misfolding.
Conclusions: Our studies used a structure-based approach to investigate α-synuclein misfolding, resulting in the creation of a powerful panel of cell lines that can be used to interrogate MSA strain biology.
Keywords: Parkinson's disease; SNCA mutations; neurodegenerative disease; protein misfolding; α-synuclein strains.
Plain language summary
In patients with Parkinson's disease (PD) and multiple system atrophy (MSA), the protein α-synuclein misfolds into distinct shapes, or strains, causing accumulation of protein aggregates in the brain. Increasing evidence indicates that the shape α-synuclein adopts determines which disease a patient will develop. As a result, it is pivotal that we understand the factors that contribute to protein misfolding in disease. In this study, we used computational modelling to predict the effect of PD-causing and novel mutations on α-synuclein misfolding into the MSA disease conformation. We then tested these predictions in cell lines expressing the same mutations to determine if α-synuclein isolated from MSA patient samples can induce protein aggregation in the presence of each mutation. Using this approach, we not only identified key mutations in the α-synuclein gene that influence the ability of the protein to misfold into the MSA strain, but we also determined the mechanism by which one of these mutations, the PD-causing E46K mutation, exerts its inhibitory effect on α-synuclein misfolding in MSA.
Conflict of interest statement
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: A.L.W. is an Editorial Board Member of this journal but was not involved in the peer-review process of this article nor had access to any information regarding its peer-review. S.H.O. and A.L.W. are the co-founders of Allagus Therapeutics, which did not contribute financial or any other support to these studies. S.H.O. and A.L.W. are inventors on U.S. Patent Application PCT/US2023/072173, which includes data reported here.
Figures

Update of
-
Structurally targeted mutagenesis identifies key residues supporting -synuclein misfolding in multiple system atrophy.bioRxiv [Preprint]. 2024 Jul 8:2024.07.04.602104. doi: 10.1101/2024.07.04.602104. bioRxiv. 2024. Update in: J Parkinsons Dis. 2024 Nov;14(8):1543-1558. doi: 10.3233/JPD-240296. PMID: 39026799 Free PMC article. Updated. Preprint.
References
-
- Postuma RB, Berg D, Stern M, et al. MDS Clinical diagnostic criteria for Parkinson’s disease. Mov Disord 2015; 30: 1591–1601. - PubMed
-
- Forster E and Lewy FH. Paralysis agitans. In: Lewandowsky M (ed) Pathologische Anatomie Handbuch der Neurologie. Berlin: Springer Verlag, 1912, pp.920–933.
-
- Papp MI, Kahn JE and Lantos PL. Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome). J Neurol Sci 1989; 94: 79–100. - PubMed
-
- Spillantini MG, Schmidt ML, Lee VM-Y, et al. α-Synuclein in Lewy bodies. Nature 1997; 388: 839–840. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

