Tau PET Imaging With [18F]MK-6240: Limited Affinity for Primary Tauopathies and High Specificity for Alzheimer's Disease
- PMID: 39957301
- PMCID: PMC11831001
- DOI: 10.1111/ene.70068
Tau PET Imaging With [18F]MK-6240: Limited Affinity for Primary Tauopathies and High Specificity for Alzheimer's Disease
Abstract
Introduction: Second-generation tau-PET tracers like [18F]MK-6240 are increasingly used both for diagnosing and quantifying Alzheimer's Disease (AD) tauopathy. However, while [18F]MK-6240 tau-PET has demonstrated excellent sensitivity for AD tauopathy, data assessing its specificity and binding in non-AD tauopathies are still scarce.
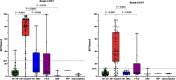
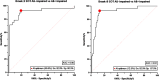
Methods: Participants were assigned to exclusive categorical diagnoses based on their amyloid (Aβ) and cognitive status. We quantified mesiotemporal (MTL) and neocortical [18F]MK-6240 tau-PET signal in 28 Aβ- cognitively impaired (CI) patients presenting various non-AD neurodegenerative disorders. Tau-PET quantifications were compared with Aβ- cognitively unimpaired (CU) subjects (n = 51) and Aβ+ CI patients (n = 77).
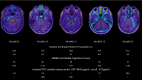
Results: Among the 28 Aβ- impaired subjects, only five presented significant and isolated mesiotemporal signal, most of them being suspected of primary age-related tauopathy (PART). Only two Aβ- impaired patients (7%) presented positive neocortical signal, both being diagnosed with fronto-temporal degeneration (FTD). The Tau-PET results of all the remaining Aβ- patients were comparable to the CU population, including eight other FTD patients. Importantly, 4R-only tauopathies (CBD and PSP) and sv-PPA were negative.
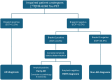
Conclusion: [18F]MK-6240 tau-PET has a special affinity for tauopathies involving 3R/4R paired helical filaments: AD, PART (Aβ- subjects with MTL-restricted tau-PET signal) and some forms of FTD while most primary tauopathies do not exhibit significant cortical signal. Positive neocortical scans are therefore highly specific for AD tauopathy. Based on those and previous results, we propose a diagnostic flowchart for MCI subjects suspected of AD or another tauopathy which may significantly reduce the need for amyloid PET or CSF measurement.
Keywords: Alzheimer's disease; MK‐6240; Tau PET; neurodegenerative diseases; tauopathy.
© 2025 The Author(s). European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

