Fecal Microbiota Transplantation Improves Cognitive Function of a Mouse Model of Alzheimer's Disease
- PMID: 39957504
- PMCID: PMC11831070
- DOI: 10.1111/cns.70259
Fecal Microbiota Transplantation Improves Cognitive Function of a Mouse Model of Alzheimer's Disease
Abstract
Background: A growing body of evidence suggests a link between the gut microbiota and Alzheimer's disease (AD), although the underlying mechanisms remain elusive. This study aimed to investigate the impact of fecal microbiota transplantation (FMT) on cognitive function in a mouse model of AD.
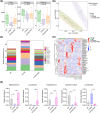
Methods: Four-month-old 5 × FAD (familial Alzheimer's disease) mice underwent antibiotic treatment to deplete their native gut microbiota. Subsequently, they received FMT either weekly or every other day. After 8 weeks, cognitive function and β-amyloid (Aβ) load were assessed through behavioral testing and pathological analysis, respectively. The composition of the gut microbiota was analyzed using 16S rRNA sequencing.
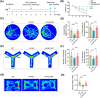
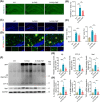
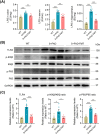
Results: Initial weekly FMT failed to alleviate memory deficits or reduce brain Aβ pathology in 5 × FAD mice. In contrast, FMT administered every other day effectively restored gut dysbiosis in 5 × FAD mice and decreased Aβ pathology and lipopolysaccharide levels in the colon and hippocampus. Mechanistically, FMT reduced the expression of amyloid β precursor protein, β-site APP cleaving enzyme 1, and presenilin-1, potentially by inhibiting the Toll-like receptor 4/inhibitor of kappa B kinase β/nuclear factor kappa-B signaling pathway. However, the cognitive benefits of FMT on 5 × FAD mice diminished over time.
Conclusion: These findings demonstrate the dose- and time-dependent efficacy of FMT in mitigating AD-like pathology, underscoring the potential of targeting the gut microbiota for AD treatment.
Keywords: Alzheimer's disease; fecal microbiota transplantation; gut microbiota; lipopolysaccharide; β‐amyloid.
© 2025 The Author(s). CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Fecal microbiota transplantation attenuates Alzheimer's disease symptoms in APP/PS1 transgenic mice via inhibition of the TLR4-MyD88-NF-κB signaling pathway-mediated inflammation.Behav Brain Funct. 2025 Jan 8;21(1):2. doi: 10.1186/s12993-024-00265-8. Behav Brain Funct. 2025. PMID: 39780269 Free PMC article.
-
Patchouli alcohol attenuates the cognitive deficits in a transgenic mouse model of Alzheimer's disease via modulating neuropathology and gut microbiota through suppressing C/EBPβ/AEP pathway.J Neuroinflammation. 2023 Jan 30;20(1):19. doi: 10.1186/s12974-023-02704-1. J Neuroinflammation. 2023. PMID: 36717922 Free PMC article.
-
Gut-derived β-amyloid: Likely a centerpiece of the gut-brain axis contributing to Alzheimer's pathogenesis.Gut Microbes. 2023 Jan-Dec;15(1):2167172. doi: 10.1080/19490976.2023.2167172. Gut Microbes. 2023. PMID: 36683147 Free PMC article.
-
Gut Microbiome in Alzheimer's Disease: from Mice to Humans.Curr Neuropharmacol. 2024;22(14):2314-2329. doi: 10.2174/1570159X22666240308090741. Curr Neuropharmacol. 2024. PMID: 39403057 Free PMC article. Review.
-
Poop for thought: Can fecal microbiome transplantation improve cognitive function in aging dogs?Open Vet J. 2025 Feb;15(2):556-564. doi: 10.5455/OVJ.2025.v15.i2.6. Epub 2025 Feb 28. Open Vet J. 2025. PMID: 40201831 Free PMC article. Review.
Cited by
-
Flavanones as Modulators of Gut Microbiota and Cognitive Function.Molecules. 2025 May 18;30(10):2203. doi: 10.3390/molecules30102203. Molecules. 2025. PMID: 40430375 Free PMC article. Review.
References
-
- “2023 Alzheimer's Disease Facts and Figures,” Alzheimer's & Dementia 19 (2023): 1598–1695. - PubMed
-
- Scheltens P., Blennow K., Breteler M. M., et al., “Alzheimer's Disease,” Lancet 388 (2016): 505–517. - PubMed
-
- Webers A., Heneka M. T., and Gleeson P. A., “The Role of Innate Immune Responses and Neuroinflammation in Amyloid Accumulation and Progression of Alzheimer's Disease,” Immunology and Cell Biology 98 (2020): 28–41. - PubMed
-
- Xin S. H., Tan L., Cao X., Yu J. T., and Tan L., “Clearance of Amyloid Beta and Tau in Alzheimer's Disease: From Mechanisms to Therapy,” Neurotoxicity Research 34 (2018): 733–748. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

