Silica Nanoparticles Loaded With Selenium Quantum Dots Reduce Myocardial Ischemia-Reperfusion Injury by Alleviating Ferroptosis and Mitochondrial Dysfunction
- PMID: 39958324
- PMCID: PMC11829639
- DOI: 10.2147/IJN.S500810
Silica Nanoparticles Loaded With Selenium Quantum Dots Reduce Myocardial Ischemia-Reperfusion Injury by Alleviating Ferroptosis and Mitochondrial Dysfunction
Abstract
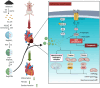
Purpose: Myocardial ischemia-reperfusion (IR) injury, a significant challenge in cardiovascular treatment, is primarily driven by ferroptosis and mitochondrial dysfunction. Despite extensive research, no clinical therapies effectively target ferroptosis in IR injury. This study aims to develop selenium-quantum-dot-loaded porous silica nanospheres (Se@PSN) as a novel therapeutic approach to address IR injury.
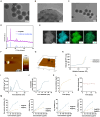
Patients and methods: Se@PSN were synthesized and tested for their reactive oxygen species (ROS) scavenging capabilities and biocompatibility. Additionally, the effects of Se@PSN on ferroptosis, mitochondrial damage, oxidative stress, and myocardial IR injury severity were evaluated.
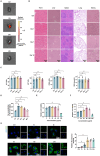
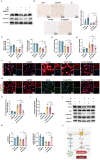
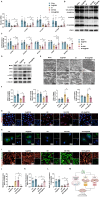
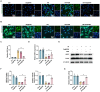
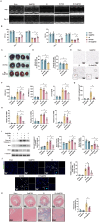
Results: Se@PSN enhanced the stability of selenium quantum dots and exhibited strong ROS scavenging abilities. Additionally, Se@PSN exhibited excellent biocompatibility. The Se@PSN treatment increased GPX4 levels, effectively inhibiting ferroptosis in cardiomyocytes. Furthermore, Se@PSN promoted the expression of mitochondrial respiratory complexes, mitigating oxidative phosphorylation damage and preserving mitochondrial function. These effects collectively resulted in reduced myocardial loss, inflammation, and fibrosis following IR injury. Compared to PSN alone, Se@PSN showed superior therapeutic efficacy against IR injury.
Conclusion: Se@PSN exhibit great potential in reducing ferroptosis and protecting mitochondrial function, making them a promising therapeutic approach for the treatment of myocardial IR injury.
Keywords: ferroptosis; myocardial ischemia reperfusion; porous silica nanospheres; reactive oxygen species; selenium.
© 2025 Li et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

Similar articles
-
Selenium-loaded porous silica nanospheres improve cardiac repair after myocardial infarction by enhancing antioxidant activity and mitophagy.Free Radic Biol Med. 2025 May;232:292-305. doi: 10.1016/j.freeradbiomed.2025.03.004. Epub 2025 Mar 5. Free Radic Biol Med. 2025. PMID: 40049339
-
Iron chelators loaded on myocardiocyte mitochondria-targeted nanozyme system for treating myocardial ischemia-reperfusion injury in mouse models.J Nanobiotechnology. 2025 Feb 15;23(1):112. doi: 10.1186/s12951-025-03197-1. J Nanobiotechnology. 2025. PMID: 39955554 Free PMC article.
-
Isoliquiritigenin alleviates myocardial ischemia-reperfusion injury by regulating the Nrf2/HO-1/SLC7a11/GPX4 axis in mice.Free Radic Biol Med. 2024 Aug 20;221:1-12. doi: 10.1016/j.freeradbiomed.2024.05.012. Epub 2024 May 9. Free Radic Biol Med. 2024. PMID: 38734270
-
Targeting Ferroptosis via Mitochondria Dynamics in Myocardial Ischemia/Reperfusion Injury.Discov Med. 2025 May;37(196):816-827. doi: 10.24976/Discov.Med.202537196.72. Discov Med. 2025. PMID: 40415357 Review.
-
Management of ROS and Regulatory Cell Death in Myocardial Ischemia-Reperfusion Injury.Mol Biotechnol. 2025 May;67(5):1765-1783. doi: 10.1007/s12033-024-01173-y. Epub 2024 Jun 9. Mol Biotechnol. 2025. PMID: 38852121 Review.
References
-
- Kajstura J, Cheng W, Reiss K, et al. Apoptotic and necrotic myocyte cell deaths are independent contributing variables of infarct size in rats. Lab Invest. 1996;74(1):86–107. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

