Outcomes with bridging radiation therapy prior to chimeric antigen receptor T-cell therapy in patients with aggressive large B-cell lymphomas
- PMID: 39958356
- PMCID: PMC11825444
- DOI: 10.3389/fimmu.2025.1517348
Outcomes with bridging radiation therapy prior to chimeric antigen receptor T-cell therapy in patients with aggressive large B-cell lymphomas
Abstract
Background: Select patients with relapsed/refractory aggressive B cell lymphoma may benefit from bridging radiation (bRT) prior to anti-CD19-directed chimeric antigen receptor T cell therapy (CAR-T). Here, we examined patient and treatment factors associated with outcomes and patterns of failure after bRT and CAR-T.
Methods: We retrospectively reviewed adults with diffuse large B-cell lymphoma (DLBCL) who received bRT prior to axicabtagene ciloleucel, tisagenlecleucel, or lisocabtagene maraleucel between 11/2017-4/2023. Clinical/treatment characteristics, response, and toxicity were extracted. Survival was modeled using Kaplan-Meier or Cox regression models for events distributed over time, or binary logistic regression for disease response. Fisher's Exact Test or Mann-Whitney U methods were used.
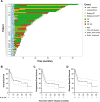
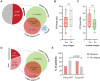
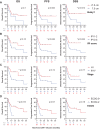
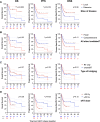
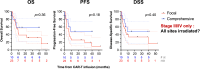
Results: Of 51 patients, 25.5% had bulky disease and 64.7% had Stage III/IV disease at the time of RT. Comprehensive bRT alone to all disease sites was delivered to 51% of patients, and 29.4% were additionally bridged with systemic therapy. Median follow-up was 10.3 months (95% CI: 7.7-16.4). Overall response rate (ORR) was 82.4% at 30 days post-CAR-T infusion. Median overall survival (OS) was 22.1 months (6.6-not reached) and the median progression-free survival (PFS) was 7.4 months (5.5-30). OS/PFS were 80% (66-99)/78% (64-87) at 1-year, and 59% (44-71)/54% (40-67) at 2-years, respectively. Comprehensive RT to all sites of disease correlated with improved PFS and OS, p ≤ 0.04. Additionally, ECOG ≥2 and Stage III/IV disease predicted poor OS (p ≤ 0.02). Disease bulk, IPI ≥3, and non-GCB histology were poor predictors for disease-specific survival (DSS), p<0.05. The latter two, as well as bRT dose of ≤30 Gy predicted worse PFS (p<0.05). Among patients with advanced stage disease, comprehensive bRT to all sites of disease (n=10) was not associated with improved OS and PFS compared to focal bRT (n=23), p>0.17. No difference was seen in bridging RT vs. chemoRT. Twenty-six patients developed relapse (50.9%), of which 46% was in-field. Risk of in-field relapse correlated with bulky disease (OR=7, 95% CI: 1.2-41, p=0.03) and lack of response at 30 day post-CAR-T evaluation (OR=16.8, 95% CI: 1.6-176, p=0.02), but not with bRT dose (p=0.27).
Conclusion: bRT and CART is a good treatment strategy for select patients with aggressive B cell lymphoma. Comprehensive bRT including all sites of disease is associated with improved outcomes.
Keywords: CAR-T; DLBCL; bridging RT; chimeric antigen receptor; radiation therapy.
Copyright © 2025 Manzar, Pinnix, Dudzinski, Marqueen, Cha, Nasr, Yoder, Rooney, Strati, Ahmed, Nze, Nair, Fayad, Wang, Nastoupil, Westin, Flowers, Neelapu, Gunther, Dabaja, Wu and Fang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. . Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. (2019) 20:31–42. doi: 10.1016/S1470-2045(18)30864-7 - DOI - PMC - PubMed
-
- Schuster SJ, Tam CS, Borchmann P, Worel N, Mcguirk JP, Holte H, et al. . Long-term clinical outcomes of tisagenlecleucel in patients with relapsed or refractory aggressive B-cell lymphomas (JULIET): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. (2021) 22:1403–15. doi: 10.1016/S1470-2045(21)00375-2 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

