Empagliflozin in Acute Myocardial Infarction Reduces No-Reflow and Preserves Cardiac Function by Preventing Endothelial Damage
- PMID: 39958474
- PMCID: PMC11830260
- DOI: 10.1016/j.jacbts.2024.08.003
Empagliflozin in Acute Myocardial Infarction Reduces No-Reflow and Preserves Cardiac Function by Preventing Endothelial Damage
Abstract
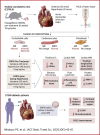
Empagliflozin treatment before acute myocardial infarction mainly targets the endothelial cell transcriptome. Empagliflozin treatment before and after myocardial infarction decreased no reflow and microvascular injury, leading to reduced infiltration of inflammatory cells, reduced infarct size, and improved cardiac function in mice. In diabetic patients receiving empagliflozin after myocardial infarction, perfused boundary region, flow-mediated dilation, and global longitudinal strain were improved.
Keywords: acute myocardial infarction; cardiac magnetic resonance; empagliflozin; microvascular injury; no-reflow.
© 2025 The Authors.
Conflict of interest statement
The authors acknowledge support of this work by the project “The Greek Research Infrastructure for Personalised Medicine (pMedGR)” (MIS 5002802) under the Action “Reinforcement of the Research and Innovation Infrastructure,” funded by the Operational Programme “Competitiveness, Entrepreneurship and Innovation” (NSRF 2014-2020) and co-financed by Greece and the European Union (European Regional Development Fund). This study was partially supported by an investigator-initiated study from Boehringer Ingelheim to Dr Andreadou. Dr Pieper is an employee of Boehringer Ingelheim Pharma & Co; and provided the research grant to the research institute of Dr Andreadou related to this work. Dr Zuurbier has received a research grant from Boehringer Ingelheim. Dr Nijveldt has received research grants from Philips Volcano and Biotronik; and speaker fees from BMS, Pfizer, and Sanofi Genzyme. Dr van Royen has received research grants from Abbott, Philips, Medtronic, and Biotronik; and speaker fees from Abbott, Bayer, RainMed, and Microport. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures







References
-
- Heusch G. Myocardial ischemia/reperfusion: translational pathophysiology of ischemic heart disease. Med. 2024;5:10–31. - PubMed
-
- Heusch G., Gersh B.J. The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: a continual challenge. Eur Heart J. 2017;38:774–784. - PubMed
-
- Heusch G. The coronary circulation as a target of cardioprotection. Circ Res. 2016;118:1643–1658. - PubMed
-
- Robbers L.F.H.J., Eerenberg E.S., Teunissen P.F.A., et al. Magnetic resonance imaging-defined areas of microvascular obstruction after acute myocardial infarction represent microvascular destruction and haemorrhage. Eur Heart J. 2013;34:2346–2353. - PubMed
LinkOut - more resources
Full Text Sources

